芥子氣經腹腔和氣管致大鼠急性肺損傷炎性反應的比較研究*
貝媛媛,朱雙雙,張長堯,趙 建,鐘玉緒,韓 瑋,劉 菲,趙玉玲,祝筱姬△
(1.濰坊醫學院研究生部,山東濰坊 261042;2.解放軍第八十九醫院呼吸科,山東濰坊 261021;3.軍事醫學科學院毒物藥物研究所,北京 100850)
?
·論著·doi:10.3969/j.issn.1671-8348.2016.26.003
芥子氣經腹腔和氣管致大鼠急性肺損傷炎性反應的比較研究*
貝媛媛1,朱雙雙1,張長堯2,趙建3,鐘玉緒3,韓瑋2,劉菲2,趙玉玲2,祝筱姬2△
(1.濰坊醫學院研究生部,山東濰坊 261042;2.解放軍第八十九醫院呼吸科,山東濰坊 261021;3.軍事醫學科學院毒物藥物研究所,北京 100850)
目的經腹腔和氣管建立大鼠芥子氣(SM)肺損傷的動物模型,比較兩種大鼠急性肺損傷模型炎性反應的差異。方法選取Sprague Dawley大鼠136只,分為5組,正常對照組8只,其他4個組(腹腔SM組、腹腔丙二醇對照組、氣管SM組、氣管丙二醇對照組)每組32只。腹腔SM組腹腔內注入稀釋的SM 0.1 mL(0.96 LD50= 8 mg/kg),氣管SM組氣管內注入稀釋的SM 0.1 mL(0.98 LD50=2 mg/kg),正常對照組不做任何處理。ELISA法檢測支氣管肺泡灌洗液和血液標本,HE染色和免疫組織化學判斷炎性反應情況。結果腹腔SM組各時間段支氣管肺泡灌洗液蛋白含量和細胞計數與氣管SM組相比顯著升高(P<0.05);腹腔SM組各時間段血清TNF-α、IL-1β、IL-6與氣管SM組相比顯著升高(P<0.05);腹腔SM組各時間段肺泡間隔T、B淋巴細胞和巨噬細胞陽性表達率與氣管SM組相比顯著增加(P<0.05)。結論大鼠在SM LD50相似的情況下,腹腔SM組支氣管肺泡灌洗液、肺泡間隔及血清炎性反應指標明顯高于氣管SM組。
芥子氣;肺/損傷;炎性反應;大鼠
芥子氣(Sulfur mustard,SM)是一種親脂性烷化劑,可迅速穿透上皮組織導致皮膚或呼吸道損傷[1-2]。皮膚、眼睛和呼吸道是SM攻擊的主要靶器官,其損傷程度與劑量和持續時間密切相關[3]。SM肺損傷早期死亡原因為肺部感染和呼吸衰竭[4]。SM可觸發促炎反應通路,炎性因子介導炎性細胞肺浸潤,并貫穿于肺損傷的全過程[5-6]。SM經皮膚、皮下、口服使小鼠染毒,以經皮膚致肺損傷的組織學改變最明顯[7]。有關SM肺損傷炎性反應的實驗指標,國內文獻報道甚少。本文通過建立經腹腔和氣管SM肺損傷大鼠模型,比較支氣管肺泡灌洗液和血清及肺泡間隔的炎性反應指標,旨在評估2種途徑SM肺損傷的差異性。
1 材料與方法
1.1材料所有動物經濰坊醫學院動物倫理委員會批準。選取健康雄性Sprague Dawley大鼠(SPF級,中國人民解放軍軍事醫學科學院實驗動物中心,合格證號:0015902)136只,體質量280~300 g,年齡15周。將大鼠分為腹腔SM組(32只)、腹腔丙二醇組(32只)、氣管SM 組(32只)、氣管丙二醇組(32只)、正常對照組(8只)。
1.2方法
1.2.1動物模式的建立SM液(純度>90%)臨用前用丙二醇稀釋至所需濃度。(1)氣管途徑染毒動物模型建立:實驗前氣管SM組和氣管丙二醇組皮下注射阿托品(0.05 mg/kg),30 min后腹腔內注射鹽酸氯胺酮(100 mg/kg)實施麻醉,氣管內注入稀釋的SM 0.1 mL(0.98 LD50=2 mg/kg),氣管丙二醇組注入丙二醇0.1 mL。(2)腹腔途徑染毒動物模型建立:同上方法實施麻醉。腹腔SM組大鼠腹腔內注入稀釋的SM 0.1 mL(0.96 LD50= 8 mg/kg),腹腔丙二醇組注入丙二醇0.1 mL,正常對照組不做任何處理。1,2-丙二醇溶液由天津致遠化學有限公司提供。
1.2.2支氣管肺泡灌洗液測定腹腔和氣管SM組大鼠,在染毒6、24、48、72 h后,腹腔注射3%戊巴比妥(30 mg/kg),麻醉后打開胸腔,心臟抽血2 mL,放血處死,然后結扎右側肺門。氣管做“T”形切口,靜脈導管(外徑1.8 mm)插入左主支氣管。抽取預熱(37.3~37.5 ℃)生理鹽水2.5 mL,緩慢注入,然后回抽灌洗液。反復抽注10次,每只大鼠灌洗5次,抽液注入離心管內(冰浴)。標本4 ℃ 離心(223.6×g離心10 min),上清液肝素抗凝,-80 ℃ 保存備用。采用全自動生化免疫一體機(COBAS 8000型,德國羅氏公司)進行蛋白含量測定。1 mL磷酸鹽緩沖液(PBS)再懸浮細胞沉淀,用臺盼藍染色,取10 μL加入細胞計數板,光鏡(BX51型,日本奧林巴斯公司)下細胞計數。
1.2.3血清炎性因子測定將腹腔SM組和氣管SM組不同時間段獲取的大鼠血2 mL,37 ℃ 水浴1 h,4 ℃ 過夜,然后223.6×g離心10 min,取上清液,分裝在無菌小瓶中,-80 ℃ 保存備用。采用酶標儀(Versa Max型,美國Molecular Devices公司),檢測血清腫瘤壞死因子α(TNF-α)、白細胞介素(IL)-1β、IL-6濃度。ELISA試劑盒由深圳科潤達生物工程有限公司提供,所有流程嚴格按說明書進行操作。
1.2.4免疫組織化學每一個標本切取15份,每5份一組進行免疫組化染色。pH 8.5,乙二胺四乙酸(EDTA)抗原修復,0.3% H2O2和山羊血清封閉,免疫組織化學采用SP法,一抗4 ℃孵育過夜(兔抗大鼠CD4單克隆抗體標記T淋巴細胞,兔抗大鼠CD20單克隆抗體標記B淋巴細胞,兔抗大鼠CD68單克隆抗體標記巨噬細胞),DAB顯色,蘇木素復染,封片。陰性對照以PBS代替一抗。CD4、CD20、CD68試劑盒由北京中杉金橋生物技術有限公司提供。
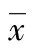
2 結 果
2.1支氣管肺泡灌洗液蛋白和細胞分析腹腔和氣管SM組支氣管肺泡灌洗液中蛋白含量和細胞計數均48 h達高峰。腹腔SM組各時間段蛋白含量和細胞計數與氣管SM組相比明顯升高(圖1A、B)。

A:支氣管肺泡灌洗液蛋白含量;B:支氣管肺泡灌洗液細胞計數;C:血清TNF-α水平;D:血清IL-1β水平; E:血清IL-6水平;F:肺泡間隔CD4陽性表達率;G:肺泡間隔CD20陽性表達率;H:肺泡間隔CD68陽性表達率。a:P<0.05,與氣管SM組比較;b:P<0.05,與正常對照組比較。
圖1大鼠支氣管肺泡灌洗液和血清及肺泡間隔炎性反應變化趨勢

a:CD4表達;b:CD20表達;c:CD68表達。A~D:6、24、48、72 h腹腔SM組陽性表達;E:正常對照組(箭頭示陽性表達,標尺為20 μm)。F~I:6、24、48、72 h氣管SM組陽性表達;J:正常對照組(箭頭示陽性表達,標尺為20 μm)。K~N:6、24、48、72 h氣管丙二醇對照組;O:正常對照組(標尺為20 μm)。
圖2大鼠肺泡間隔T淋巴細胞、B淋巴細胞、巨噬細胞表達(×400)
2.2血清炎性因子分析腹腔和氣管SM組血清TNF-α、IL-1β、IL-6水平24 h達高峰,腹腔SM組各時間段血清炎性因子水平與氣管SM組相比明顯升高(圖1C~E)。
2.3大鼠肺泡間隔炎細胞浸潤
2.3.1腹腔SM組(CD4)6、24、48 h 肺泡間隔T淋巴細胞聚集成簇,72 h呈團簇狀。氣管SM組(CD4)6、24、48、72 h肺泡間隔T淋巴細胞聚集成簇。丙二醇和正常對照組(CD4)呈零星分布(圖2a A~O)。腹腔SM組各時間段肺泡間隔T淋巴細胞陽性表達率與氣管SM組相比明顯增多(圖1F)。
2.3.2腹腔和氣管SM組(CD20)6 h肺泡間隔B淋巴細胞呈帶狀分布,24、48、72 h聚集成簇。丙二醇和正常對照組呈零星分布(圖2b A~O)。腹腔SM組各時間段肺泡間隔B淋巴細胞陽性表達率與氣管SM組相比明顯增多(圖1G)。
2.3.3腹腔和氣管SM組(CD68)6 h 肺泡間隔巨噬細胞呈散在分布,24 h增多,48、72 h明顯增多。丙二醇和正常對照組呈零星分布(圖2c A~O)。腹腔SM組各時間段肺泡間隔巨噬細胞陽性表達率與氣管SM組相比明顯增多(圖1H)。
3 討 論
SM誘導肺損傷涉及炎性介質和炎性細胞反應。Mcclintock等[8]研究發現,大鼠氣管內滴注2-氯乙基乙基硫醚(CEES)6 mg/kg,24 h肺泡內可發生出血、水腫、巨噬細胞和單核細胞聚集。另有學者發現,CEES可誘導促炎因子IL-6 和 IL-1β上調,同時轉錄因子血清加速因子-1(serum accelerator factor-1,SAF-1)/癌基因相關鋅指蛋白(myc-associated zinc finger protein,MAZ)活性增加[9]。豚鼠CEES染毒后檢測血清發現,24 h 血清TNF-α、IL-1β、IL-6、IL-8水平升高[10]。可見,在SM誘導機體應激狀態下,炎性細胞能釋放促炎介質和細胞因子,刺激中性粒細胞的溢出和集聚[11-13]。在損傷部位,中性粒細胞也可通過脫顆粒和髓過氧化物酶的釋放來改變組織的微環境[14]。
本研究發現,腹腔和氣管SM組炎性反應指標的變化具有如下特點:(1)支氣管肺泡灌洗液蛋白含量和細胞計數48 h 達高峰;(2)血清促炎因子TNF-α、IL-1β、IL-6水平24 h 達高峰;(3)免疫組織化學顯示肺泡間隔T、B淋巴細胞和巨噬細胞浸潤隨時間延長增多;(4)上述炎性反應指標腹腔SM組與氣管SM組相比明顯升高。本研究支氣管肺泡灌洗液中蛋白含量和細胞計數與Anderson 等[15]和Calvet 等[16]報道一致。促炎因子水平與Yego 等[10]和Emad 等[17]報道一致,與Yaraee 等[18]和Pourfarzam 等[19]報道相反。筆者認為,支氣管肺泡灌洗液蛋白含量和細胞計數增多,可能與肺間質毛細血管和肺上皮細胞通透性增加有關,屬一種肺實質伴隨肺結構改變的炎性反應。本研究還發現,SM致急性肺損傷炎細胞浸潤以淋巴細胞為主,這與文獻報道以中性粒細胞和巨噬細胞浸潤為主不一致[20]。分析可能與SM誘導細胞死亡,促炎介質(TNF-α,IL-6,IL-1β,IL-8等)釋放到細胞外基質中,激活巨噬細胞和肥大細胞,啟動免疫反應有關。與此同時,炎性細胞能釋放促炎介質和化學引物,在損傷部位刺激中性粒細胞溢出與集聚[13,21]。本研究還顯示,兩種途徑和濃度SM致急性肺損傷動物模型,肺泡間隔有大量淋巴細胞浸潤,中量巨噬細胞浸潤,其肺損傷程度與時間和細胞密度相關。這表明SM誘導急性肺損傷免疫反應和炎性反應共存,以免疫反應為主導。文獻[22]報道,SM腹腔注射引起的肺損傷比經皮下注射或口服途徑更嚴重。當大鼠經腹腔注射SM劑量高于10 mg/kg時,就會出現大鼠死亡[23]。有學者發現,大鼠SM氣管內吸入劑量(1.4 mg/kg),可產生明顯的肺臟炎性反應[24]。所以,在預期實驗設計的基礎上選擇SM劑量(0.96 LD50=8 mg/kg)腹腔造模和(0.98 LD50=2 mg/kg)氣管造模。本研究提示,大鼠在SM LD50相似的情況下,SM經腹腔染毒肺炎性反應指標比經氣管明顯升高。分析大鼠腹膜腔的腹膜對SM的接觸和吸收遠遠大于氣管的黏膜,由此存在毒素吸收入血的濃度差異,且SM的劑量與組織和血的炎性反應程度呈正相關。SM腹腔染毒致大鼠急性肺損傷炎性反應重,推測可能與腹膜腔對SM的快速吸收,血中SM的濃度迅速升高有關。在未來的戰爭和恐怖事件中,很難預測SM的染毒方式和劑量。本研究闡述的SM相關機制與獲得的參數,可為SM的預防與治療提供借鑒。
[1]Chilcott RP,Jenner J,Carrick W,et al.Human skin absorption of Bis-2-(chloroethyl)sulphide (sulphur mustard) in vitro[J].J Appl Toxicol,2001,20(5):349-355.
[2]Kumar P,Gautam A,Prakash CJ,et al.Ameliorative effect of DRDE 07 and its analogues on the systemic toxicity of sulphur mustard and Nitrogen mustard in rabbit[J].Hum Exp Toxicol,2010,29(9):747-755.
[3]Kehe K,Balszuweit F,Emmler J,et al.Sulfur mustard research--strategies for the development of improved medical therapy[J].Eplasty,2008(8):32.
[4]Kehe K,Szinicz L.Medical aspects of sulphur mustard poisoning[J].Toxicology,2005,214(3):198-209.
[5]Anderson DR,Holmes WW,Lee RB,et al.Sulfur mustard-induced neutropenia:treatment with granulocyte colony-stimulating factor[J].Mil Med,2006,171(5):448-453.
[6]Anderson DR,Taylor SL,Fetterer DP,et al.Evaluation of protease inhibitors and an antioxidant for treatment of Sulfur mustard-induced toxic lung injury[J].Toxicology,2009,263(1):41-46.
[7]Vijayaraghavan R,Kulkarni A,Pant SC,et al.Differential toxicity of Sulfur mustard administered through percutaneous,subcutaneous,and oral routes[J].Toxicol Appl Pharmacol,2005,202(2):180-188.
[8]Mcclintock SD,Hoesel LM,Das SK,et al.Attenuation of half Sulfur mustard gas-induced acute lung injury in rats[J].J Appl Toxicol,2006,26(2):126-131.
[9]Mukhopadhyay S,Mukherjee S,Ray BK,et al.Antioxidant liposomes protect against CEES-induced lung injury by decreasing SAF-1/MAZ-mediated inflammation in the Guinea pig lung[J].J Biochem Mol Toxicol,2010,24(3):187-194.
[10]Yego EC,Dillman JF.Cytokine regulation by MAPK activated kinase 2 in keratinocytes exposed to Sulfur mustard[J].Toxicol In Vitro,2013,27(7):2067-2075.
[11]Eming SA,Krieg T,Davidson JM.Inflammation in wound repair:Molecular and cellular mechanisms[J].J Invest Dermatol,2007,127(3):514-525.
[12]Nathan C.Neutrophils and immunity:challenges and opportunities[J].Nat Rev Immunol,2006,6(3):173-182.
[13]SilvaMT.Whentwoisbetterthanone:macrophagesandneutrophilsworkinconcertininnateimmunityascomplementaryandcooperativepartnersofa myeloid phagocyte system[J].J Leukoc Biol,2010,87(1):93-106.
[14]Lefkowitz DL,Lefkowitz SS.Macrophage-neutrophil interaction:a paradigm for chronic inflammation revisited[J].Immunol Cell Biol,2001,79(5):502-506.
[15]Anderson DR,Byers SL,Vesely KR.Treatment of Sulfur mustard (HD)-induced lung injury[J].J Appl Toxicol,2000,20(Suppl 1):S129-S132.
[16]Calvet JH,Planus E,Rouet P,et al.Matrix metalloproteinase gelatinases in Sulfur mustard-induced acute airway injury in Guinea pigs[J].Am J Physiol,1999,276(5 Pt 1):L754-L762.
[17]Emad A,Emad Y.CD4/CD8 ratio and cytokine levels of the BAL fluid in patients with bronchiectasis caused by Sulfur mustard gas inhalation[J].J Inflamm (Lond),2007,4(2):2.
[18]Yaraee R,Hassan ZM,Pourfarzam S,et al.Fibrinogen and inflammatory cytokines in spontaneous sputum of sulfur-mustard-exposed civilians--Sardasht-Iran Cohort Study[J].Int Immunopharmacol,2013,17(3):968-973.
[19]Pourfarzam S,Ghazanfari T,Yaraee R,et al.Serum levels of IL-8 and IL-6 in the long term pulmonary complications induced by Sulfur mustard:Sardasht-Iran Cohort Study[J].Int Immunopharmacol,2009,9(13/14):1482-1488.
[20]Gao X,Ray R,Xiao Y,et al.Macrolide antibiotics improve chemotactic and phagocytic capacity as well as reduce inflammation in Sulfur mustard-exposed monocytes[J].Pulm Pharmacol Ther,2010,23(2):97-106.
[21]Jaeschke H.Mechanisms of liver injury.II.mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions[J].Am J Physiol Gastrointest Liver Physiol,2006,290(6):G1083-G1088.
[22]Weinberger B,Laskin JD,Sunil VR,et al.Sulfur mustard-induced pulmonary injury:therapeutic approaches to mitigating toxicity[J].Pulm Pharmacol Ther,2011,24(1):92-99.
[23]Jafari M.Dose- and time-dependent effects of Sulfur mustard on antioxidant system in liver and brain of rat[J].Toxicology,2007,231(1):30-39.
[24]Malaviya R,Sunil VR,Cervelli J,et al.Inflammatory effects of inhaled Sulfur mustard in rat lung[J].Toxicol Appl Pharmacol,2010,248(2):89-99.
A comparative study on inflammatory response due to sulfur mustard-induced acute lung injury in rat via the intraperitoneal and tracheal injection*
Bei Yuanyuan1,Zhu Shuangshuang1,Zhang Changyao2,Zhao Jian3,Zhong Yuxu3,HanWei2,LiuFei2,ZhaoYuling2,ZhuXiaoji2△
(1.DepartmentofGraduate,WeifangMedicalUniversity,Weifang,Shandong261042,China;2.DepartmentofRespiration,The89thHospitalofPLA,Weifang,Shandong261021,China;3.InstituteofPharmacologyandToxicology,AcademyofMilitaryMedicalSciences,Beijing100850,China)
ObjectiveThe purpose of this study was to establish animal model of sulfur mustard (SM)-induced acute lung injury in rats via the intraperitoneal and the tracheal injection,in order to compare the difference of inflammatory reaction.Methods136 male Sprague Dawley rats were selected,then were randomly divided into the five groups,the control group with 8 cases,other four groups (i.e.the intraperitoneal SM group,the intraperitoneal propylene glycol group,the tracheal SM group,the tracheal propylene glycol group) with 32 cases in each group.The intraperitoneal SM group were injected intraperitoneally with diluted SM 0.1 mL(0.96 LD50=8 mg/kg),the tracheal SM group were injected intratracheally with diluted SM 0.1 mL(0.98 LD50= 2 mg/kg),meanwhile the status quo was kept with the normal group.SM-induced inflammatory reaction was observed by bronchoalveolar lavage fluid (BALF),serum examination,Hematoxylin Eosin staining,and immunohistochemical staining.ResultsCompared with the tracheal SM group at different time,protein contents and cell counts of BALF in the intraperitoneal SM group were significantly inceased,respectively (P<0.05).Compared with the tracheal SM group at different time,the levels of serum TNF-α,IL-1β,IL-6 in the intraperitoneal SM group were significantly inceased,respectively (P<0.05).The positive expression ratio of T lymphocytes,B lymphocytes and macrophages in intraperitoneal SM group at different time were increased compared with the tracheal SM group,respectively (P<0.05).ConclusionUnder similar SM LD50in rat,in the intraperitoneal SM group,inflammatory reaction of BALF,alveolar septum,and serum were significantly higher than in the tracheal SM group.
mustard gas;lung/injuryies;inflammatory reaction;rat
國家“重大新藥創制”科技重大專項(2013ZX09J13013-01B)。作者簡介:貝媛媛(1991-),在讀研究生,主要從事呼吸毒理學研究。△
,E-mail:xiaojizhu@163.com 。
R114
A
1671-8348(2016)26-3608-03
2016-03-18
2016-06-01)

