銀-烯配位的銀ガ配合物的合成、晶體與熒光性質(zhì)
劉振香 陳 鋆 羅小會 刁銀軍 吳昌勝
(金華職業(yè)技術(shù)學(xué)院制藥與材料工程學(xué)院,金華 321017)
0 Introduction
Metallosupramolecular chemistry involves the use of bridging organic ligands and metal ions for the construction of discrete and polymeric assemblies with diverse molecular architectures[1].There has been rapid and popular development of Agガcomplexes in recent years,as its structural versatility in its coordination chemistry[2].First,Agガ is able to form bonds with different donors simultaneously,which readily binds to nitrogen heterocycles,as well as to ligands having oxygen,phosphorus,sulfur and other common donors[3-8],and Agガhas long been known to interact with carboncarbon double bonds[1],too.Second,the coordination geometries of Agガare a wide variety and offer particularly versatile structures.Third,the d10-d10interactions between two close-shells of Agガatom may exist in the complexes of Agガ,increasing the possibility of forming complicated multi-dimensional systems[9].So Agガ is a favorable building block or connecting node for coordination polymers,which have special photophysical and photochemical properties[10].
Furthermore,luminescent compounds composed of d10metal centers and organic ligands are of great interest because of their potential applications in the areas of chemical sensors and photoluminescent properties[11],such as light-emitting diodes(LEDs)[12],sensors,photochemistry[13].
Herein,4-allyl-2-methoxyphenyl nicotinate was chosen to be the ligand to generate metallacyclic ensembles because of its structures of the pyridine ring,which has the better coordination ability,and the carbon-carbon double bonds,which can interact with Agガ to form the interesting metal organic complexes.
1 Experimental
1.1 Materials
All reagents and solvents employed were commercially available and used without further purification,except acetone and dichloromethane were used after dried by MgSO4.
1.2 Physical measurement
Powder X-ray diffraction measurements were carried out with a Bruker D8 Focus X-ray diffractometer to check the phase purity.The C,H and N microanalyses were performed with Perkin Elmer 2400 Ⅱ CHNO/S elemental analyzer.The FTIR spectra were recorded from KBr pellets in the range of 4 000~400 cm-1on a Shimadzu FTIR-8900 spectrometer.Thermogravimetric measurements were carried out from R.T.to 900 ℃ on pre weighed samples in nitrogen stream using a Seiko Exstar 6000 TG/DTA 6300 apparatus with a heating rate of 10℃·min-1.The fluorescence on solid samples were determined with a RF-5301PCspectrophotometer.
1.3 Preparation of ligands and complex
1.3.1 Synthesis of 4-allyl-2-methoxyphenyl nicotinate(L)
The ligand L(4-allyl-2-methoxyphenyl nicotinate)was synthesized according to the literature[14].Thionyl chloride(150 mL)was added to 3-pyridine carboxylic acids(0.65 mol,80 g).The mixture was stirred and refluxed for 3 h.The thionyl chloride was then evaporated under reduced pressure giving a less yellow crystalline residue of nicotinoyl hydrochloride.The purity of nicotinoyl hydrochloride was quite sufficient to use the product directly for the following synthesis.
A mixture solution of dry triethylamine (30 mL)and CH2Cl2(100 mL)containing eugenol(16.4 g,0.1 mol)was added dropwisely to a stirred solution of nicotinoyl hydrochloride (17.8 g,0.1 mol)in dry dichloromethane(50 mL)at room temperature.After 3 h,to the mixture,saturated sodium carbonate was added dropwisely to adjust the pH value to about 7.0.The CH2Cl2layer was washed successively with saturated NaCl solution and H2O for three times.The CH2Cl2layer was dried by anhydrous MgSO4and the solvent was removed under reduced pressure.Recrystallization in ethanol gave white prism crystals.Yield:16.1 g(60%).m.p.70~72 ℃.Anal.Calcd.for C16H15NO3(%):C,71.36;H,5.61;N,5.20 .Found(%):C,71.35,H,5.62,N,5.21.IR (KBr,cm-1):3 130(s,br),1 745(vs),1 642(m),1 606(s),1 589(s),1 508(s),1 464(s),995(m),911(m),734(s).1H NMR(400 MHz,CDCl3):δ3.39 (d,2,-CH2-),3.80(s,3,-OCH3),5.09~5.14(m,2,H2C=),5.92~6.03(m,1,=CH),6.81(d,1,H-5),6.84(s,1,H-3),7.06 (d,1,H-6),7.42~7.45(dd,1,H-5′),8.45(d,1,H-4′),8.83(d,H-6′),9.39(s,1,H-2′).
1.3.2 Synthesis of[AgL(NO3)]n(1)
A solution of AgNO3(16.9 mg,0.1 mmol)in 4.0mL water was slowly added to a solution of 4-allyl-2-methoxyphenyl nicotinate(L)(26.0 mg,0.1 mmol)in THF (6 mL).Colorless single crystals suitable for X-ray analysis were obtained after a few days with 82%yield(based on L).Anal.Calcd.for C16H15N2O6Ag(%):C,43.76;H,3.44;N,6.38.Found(%):C,43.80,H,3.43,N,6.40.IR(KBr,cm-1):3 448(br),1 736(s,br),1 745(s),733(s).
1.4 X-ray crystallography
Crystallographic data were collected on a Rigaku R-Axis Rapid IP X-ray diffractometer with graphitemonochromated Mo Kα radiation (λ=0.071 073 nm).The data were corrected for Lp and absorption effects.The structures were solved by direct methods and expanded with difference Fourier techniques.All nonhydrogen atoms were refined anisotropically in fullmatrix least-squares refinements based on F2.All hydrogen atoms were generated geometrically.The C2 atom of allyl in L was disordered and the occupancies were rened to 1∶1.07,respectively.All calculations were performed with SHELXTL-97 packages[15].The crystal data and structure refinements are summarized in Table 1,and the selected bond lengths and bond angles are listed in Table 2.
CCDC:985940,L;985941,1.
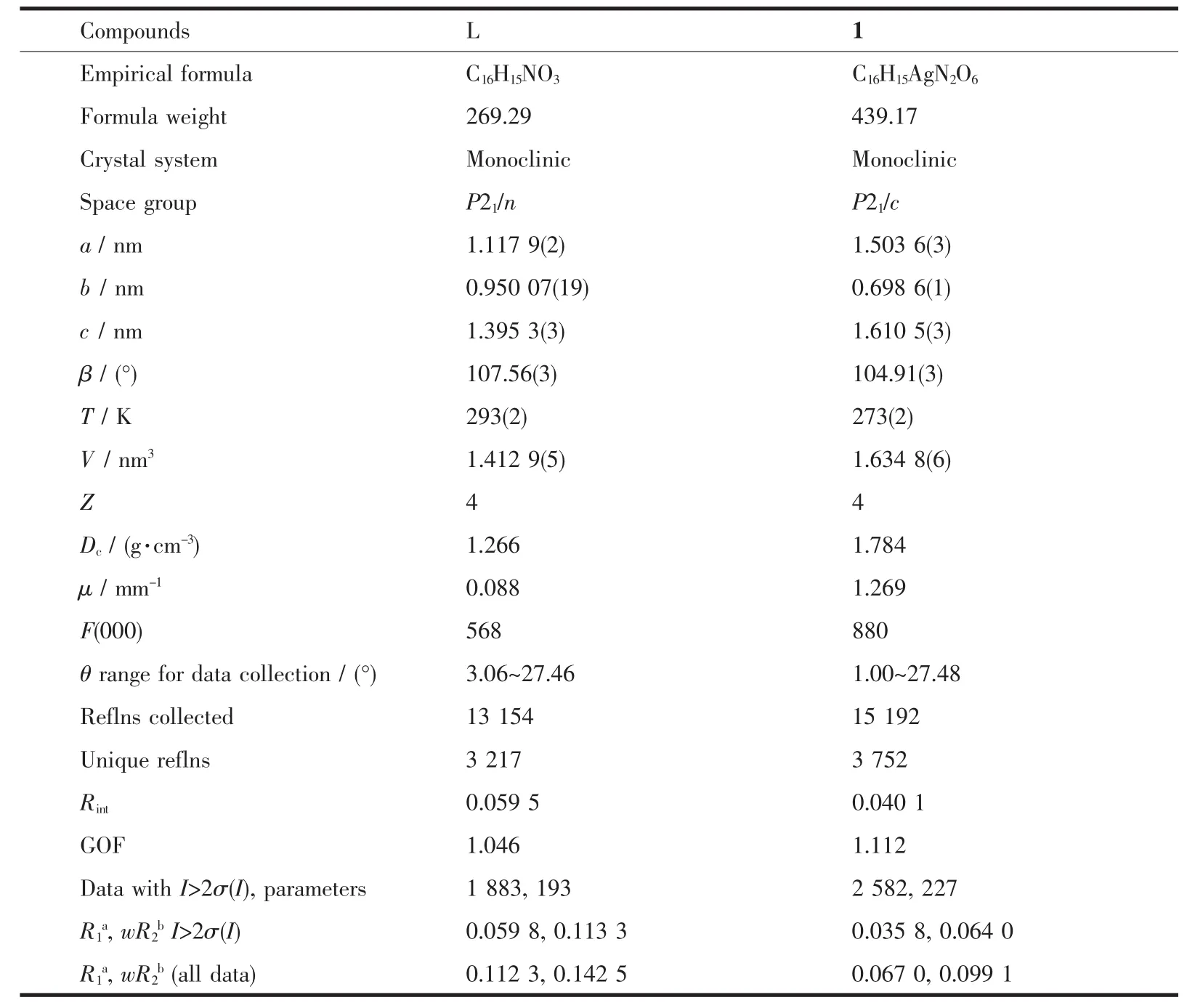
Table 1 Crystal data and structure and refinement for compounds L and 1
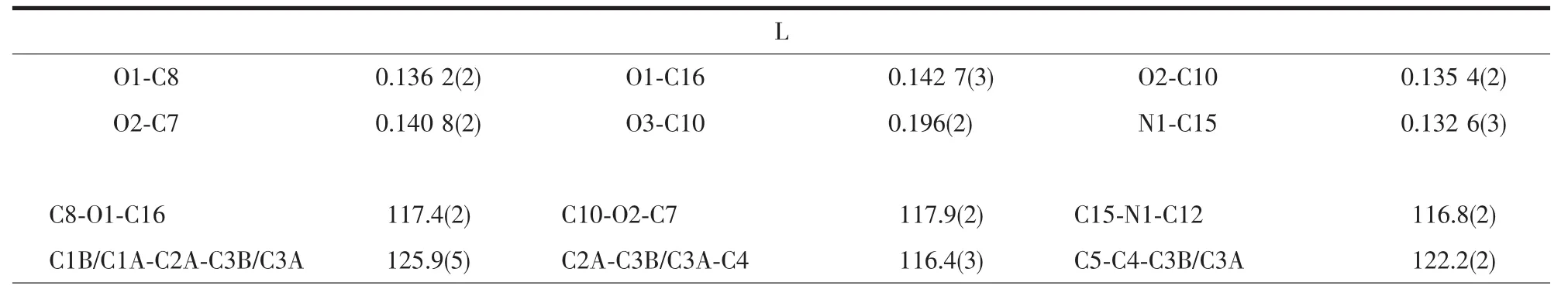
Table 2 Selected bond lengths(nm)and angles(°)for L and 1
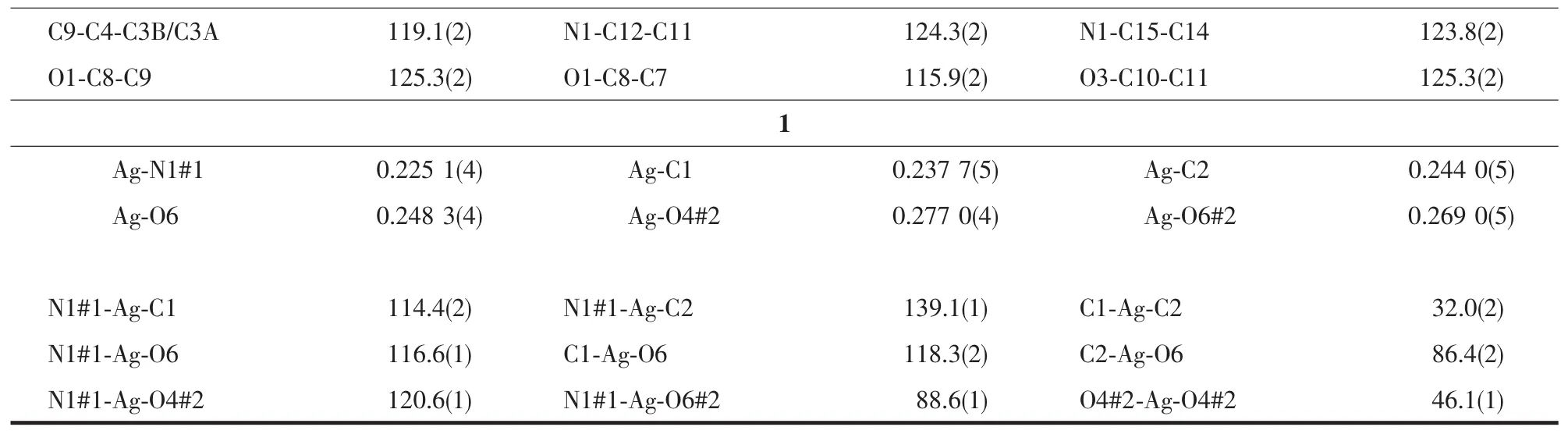
Continued Table 2
2 Results and discussion
2.1 Crystal structures
The molecular structure of compound L is illustrated in Fig.1.L crystallizes in the monoclinic space group P21/n and the mean planes through the benzyl and pyridine units make a dihedral angle of 75.6(1)°.
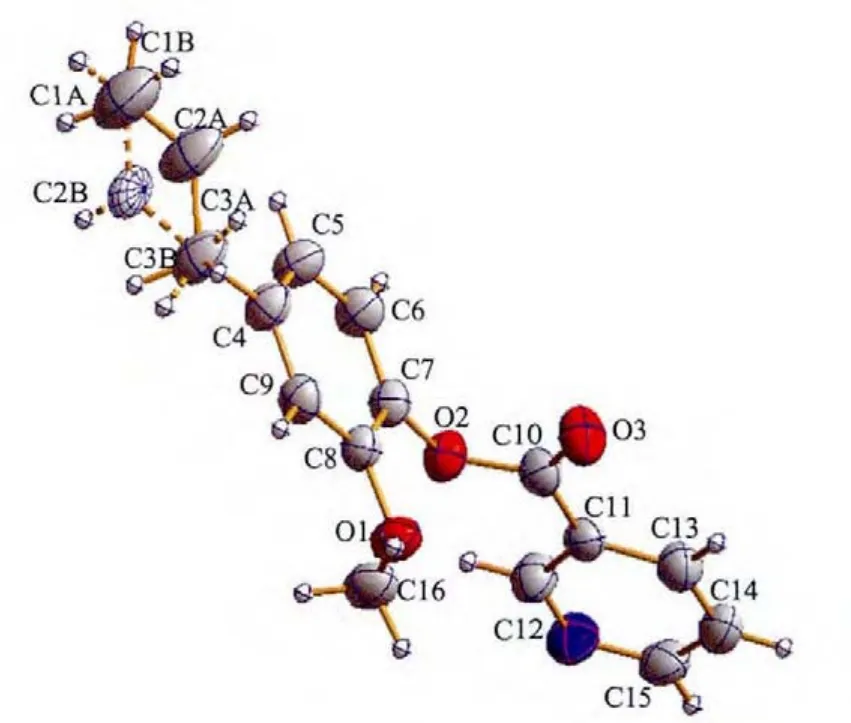
Fig.1 ORTEPview of compound L with ellipsoids at 35%probability levels
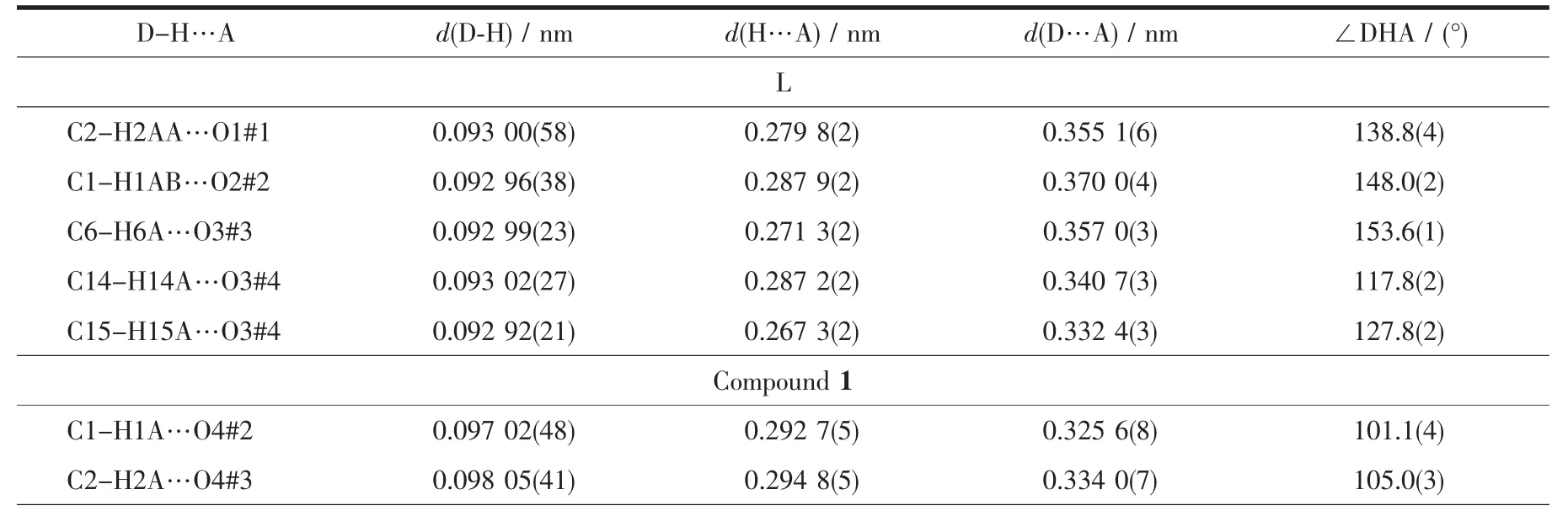
Table 3 C-H…O hydrogen bonds for L and 1
The association of molecules to form crystals is effected by the C-H…O interactions(documented in Table 3).In this structure,there appear to be three types of C-H…O interactions.The first type is C2…H2AA…O1#1 hydrogen bond interaction to form a dimer(Fig.2a).The second type is to form a 2D layer structure involving C1-H1AB…O2#2 hydrogen bonding interactions at the ends of the dimer(Fig.2b).The third type also is a 2D layer(Fig.2c)but involves two modes of C-H…O interactions,C6-H6A…O3#3 as well as C14-H14A(H15A)…O3#4 and C15-H15A…O3#4,so one molecule links four different molecules through the C-H…O3 interactions and at last forming a 3D supramolecular structure(Fig.2d).
The structure of compound 1 is shown in Fig.3.The Agガatom is four-coordinated by one oxygen(O6)of nitrate anion,two carbon atoms (C1,C2)and one nitrogen (N1)from different ligands.The mean planes through the benzyl and pyridine units make a dihedral angle of 74.9(1)°,which is smaller than L.
The Ag-N1 bond distance is 0.225 4(3)nm,whichis comparable to those previously reported Agガ-Npycoordination distances[16-18].The silver ion is coordinated by the C1-C2 double bond of one molecule with Ag-C bond distancesrangingfrom0.237 9(4)nmto0.244 3(4)nm.The Ag-C distance is similar to those of the reported silver complexes with alkenes and arenes[19-21],
whereas the C1-C2 double bond is consistent with the IR spectrum which suggests the C=C stretching vibrations of the ligand.The elongation of olefinic bond is mainly observed in the basal ligands with the C1-C2 distance of 0.134 3(5)nm.

Continued Table 3
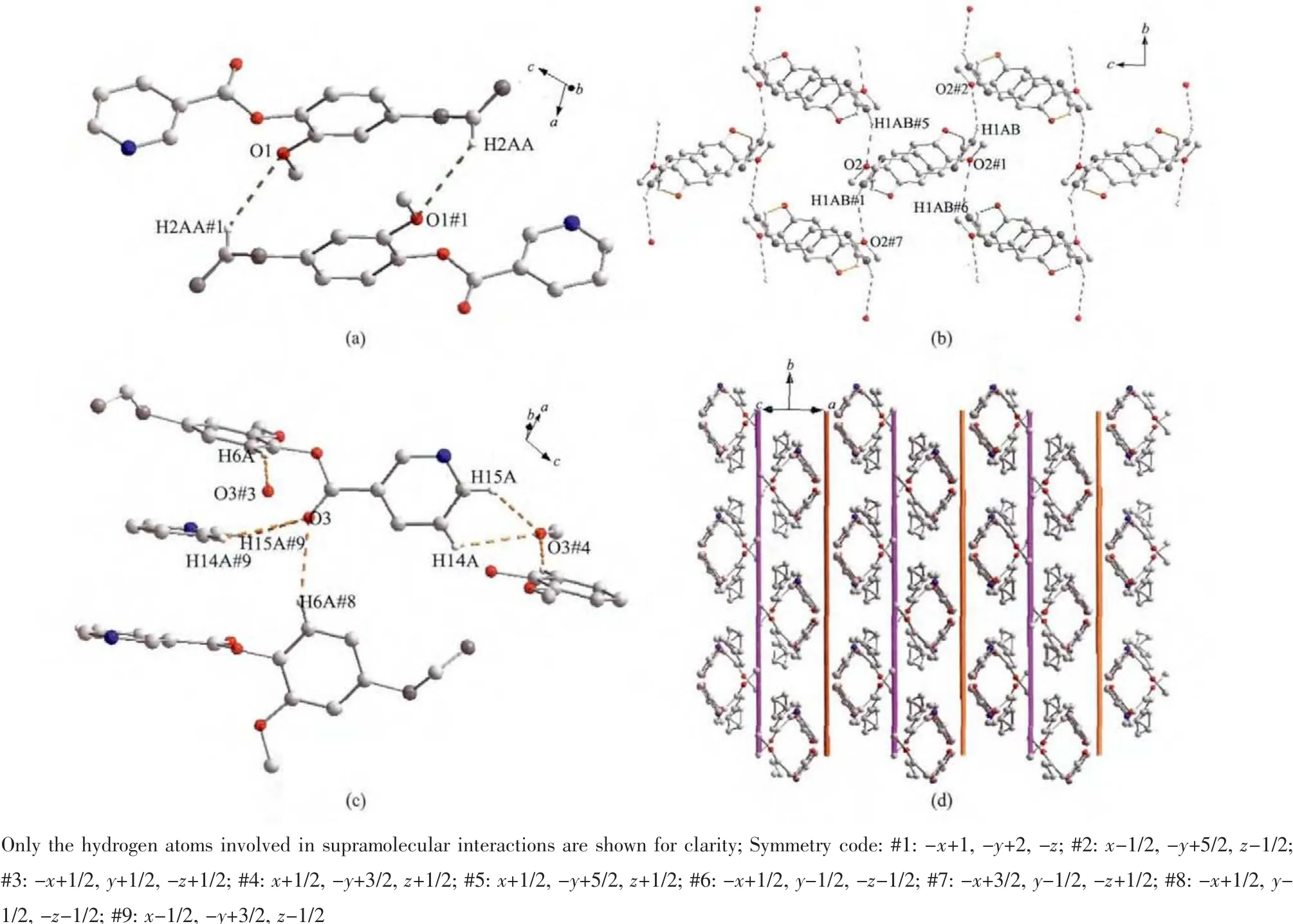
Fig.2 (a)Dimer stepped motif formed with the C2-H2AA…O1 hydrogen bonding interaction;(b)2D layers formed by the C1-H1AB…O2 hydrogen bonding interaction(the pyridine rings were omitted for clarity);(c)2D layers formed by the C-H…O3 hydrogen bonding interaction;(d)View of the 3D structure of L
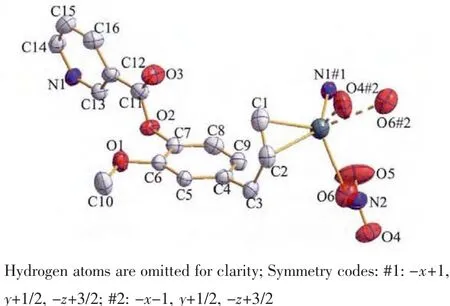
Fig.3 ORTEPview of coordination environment of Ag ions and atomic labeling in 1 with ellipsoids at 35%probability levels
The Ag…O4#2(O6#2)distance of 0.268 8(3)(0.277 4(3))nm is shorter than the van der Waals contact distance of 0.324 nm.Therefore,the Ag…O distances suggest non-negligible interactions between Agガand NO3-group,which may be described as a semi-coordination mode.Hence Agガcan be regarded as a pseudo-six-coordinated environment(Fig.3).The L ligand connects the Agガions together by N atoms and allyl groups to generate a 1D left-handed helical chain of[AgL]∞around the crystallographic 21screw axis with the pitch of 0.698 6(1)nm along the b axis.And the NO3-group connects Agガions of adjacent chains together by the Ag…O weak interactions to generate a 1D right-handed helical chain of[AgNO3]∞around the crystallographic 21screw axis with the pitch of 0.698 6(1)nm along the b axis.Thus two kinds of alternated helices are connected in…ABAB…fashion to form a 2D achiral layer(Fig.4).
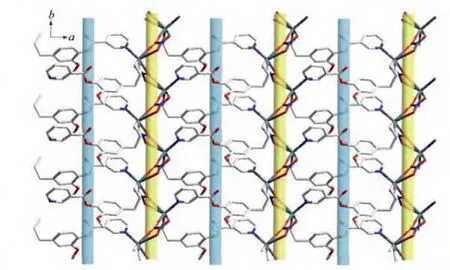
Fig.4 View of two-dimensional layer comprising the right(yellow)-and left(green)-handed helical chains
The resulted 2D layers are further connected to form the 3D supramolecular architecture due to weak C-H…Ohydrogen bonds(Table 3).
2.2 Fluorescence properties
The photoluminescence properties of compounds L and 1 were studied in the solid state at room temperature.As shown in Fig.5,L and 1 show the similar emission and excitation spectra,which exhibit luminescence in violet region with centers atλmax=364 nm (λex=334 nm).These emissions can be attributed to neither metal-to-1igand charge transfer(MLCT)nor ligand-to-metal charge transfer (LMCT)because the Agガ ion has d10configuration with one positive charges.The d-orbitals are contracted and therefore the electrons in these orbitals are much less accessible for back bonding to p-acceptor ligands,and silver cation has a weak electro accepting nature with respect to electrons from L.So the luminescent emissions of 1 is from the ligand,which can be ascribed to the intraligand π*-π transitions[10,22-23].
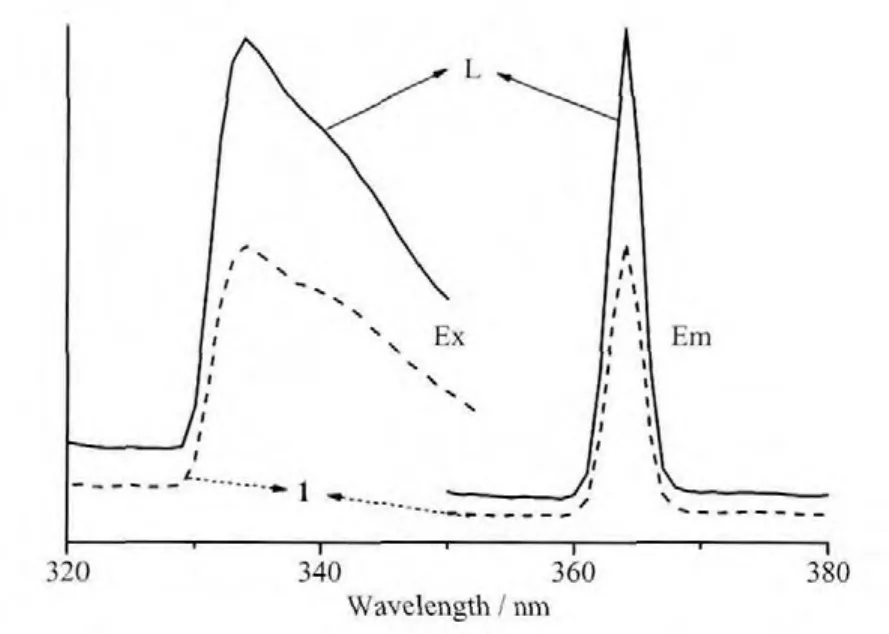
Fig.5 Emission and excitation spectra for compounds L and 1
3 Conclusions
In summary,Silver-containing complex 1 was synthesized through the interactions of 4-allyl-2-methoxyphenyl nicotinate (L)and silver nitrate.Two new compounds L and 1 were confirmed by single crystal X-ray diffraction study.
The observations of the luminescent properties indicate that the two complexes showed violet-light photoluminescent,but 1 may be excellent candidate of photoactive materials,which are thermally stable and insoluble in common organic solvents.
Acknowledgements:The authors would like to thank Prof.Dr.ZHENG Yue-Qing for initiating our interest in this work and Dr.XU Wei and ZHU Hong-Lin for their comments on the manuscript.Acknowledgement is made to the classmates WAN Dan-Weng,WEN Gang-Zhu et al.for the helps and X-ray diffraction measurements.
[1]Burgess J,Cottam J,Steel P.Aust.J.Chem.,2006,59(5):295-297
[2]Holloway C,Melnik M,Nevin W,et al.J.Coord.Chem.,1995,35:85-178
[3]Liu H K,Hu J,Wang T W,et al.J.Chem.Soc.,Dalton Trans.,2001:3534-3540
[4]Cui Y,He C.J.Am.Chem.Soc.,2003,125:16202-16203
[5]Bowmaker G,Hanna J,Skelton B,et al.J.Chem.Soc.,Dalton Trans.,2012,41:5409-5417
[6]Yin X,Xie M B,Zhang W G,et al.Acta Crystallogr.Sect.E:Struct.Rep.Online,2007,E63:m2273
[7]Sagatys D,Smith G,Bott R,et al.Polyhedron,1993,12(6):709-713
[8]Bu X H,Hou W F,Du M,et al.Cryst.Growth Des.,2002,2(4):303-307
[9]Chen J,Hou L,Zhang Y N,et al.Inorg.Chem.Commun.,2012,24:73-76
[10]Dang D B,Zheng Y N,Bai Y,et al.Cryst.Growth Des.,2012,12:3856-3867
[11]Etaiw H,Badr B.J.Inorg.Organomet.Polym.Mater.,2011,22:478-491
[12]Brito I,Vallejos J.Cárdenas A,et al.Inorg.Chem.Commun.,2011,14:897-901
[13]Pochodylo A,LaDuca R.Inorg.Chim.Acta,2012,389:191-201
[14]Sadeghian H,Seyedi S M,Saberi M R,et al.Bioorg.Med.Chem.,2008,16:890-901
[15]Sheldrick G M.SHELXS-97,Program for Crystal Stucture Refinement,and SHELXL-97,Program for Crystal Stucture Solution,G?ttingen University,Germany,1997.
[16]Farnum G,Knapp W,LaDuca R.Polyhedron,2009,28:291-299
[17]Meyer G,Berners A,Pantenburg I.Z.Anorg.Allg.Chem.,2006,632:34-35
[18]Ma M L,Li X Y,Zhao X L,et al.CrystEngComm,2011,13:1752
[19]Kelemu S,Steel P.Cryst.Growth Des.,2014,14:1245-1250
[20]Little M,Halcrow M,Harding L,et al.Inorg.Chem.,2010,49:9486-9496
[21]Kuwatani Y,Yoshida T,Hara K,et al.Org.Lett.,2000,2:4017-4020
[22]ZHUN Xiao-Yan(朱小燕),SONG Hui-Hua(宋會花),HE Rong(何蓉),et al.Chinese J.Inorg.Chem.(無機(jī)化學(xué)學(xué)報),2012,28(9):2011-2016
[23]DENGYI-Fang(鄧奕芳),TANXiong-Wen(譚雄文),ZHANG Chun-Hua(張春華),et al.Chinese J.Inorg.Chem.(無機(jī)化學(xué)學(xué)報),2010,26(5):909-912