某院419例藥品不良反應(yīng)報(bào)告分析
周偉 馬天宇 娜榮華 代亞軍
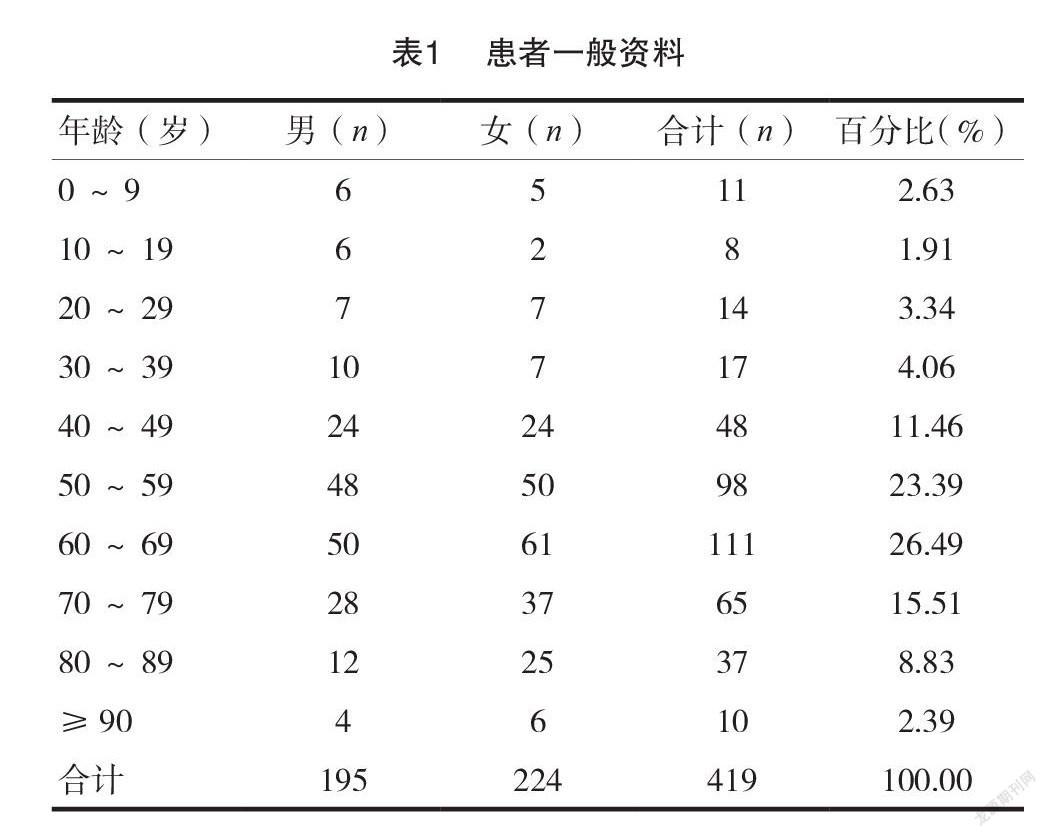
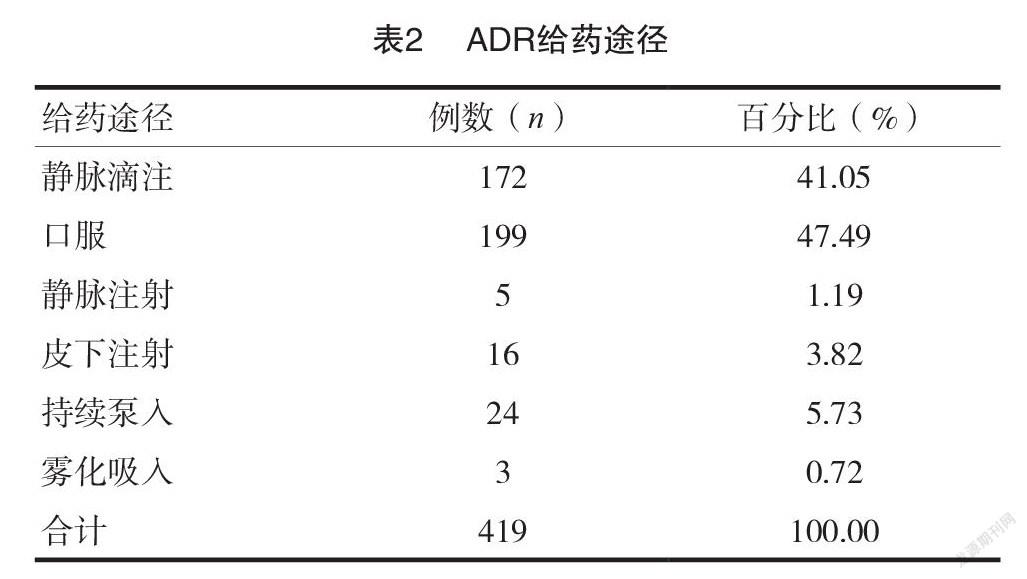
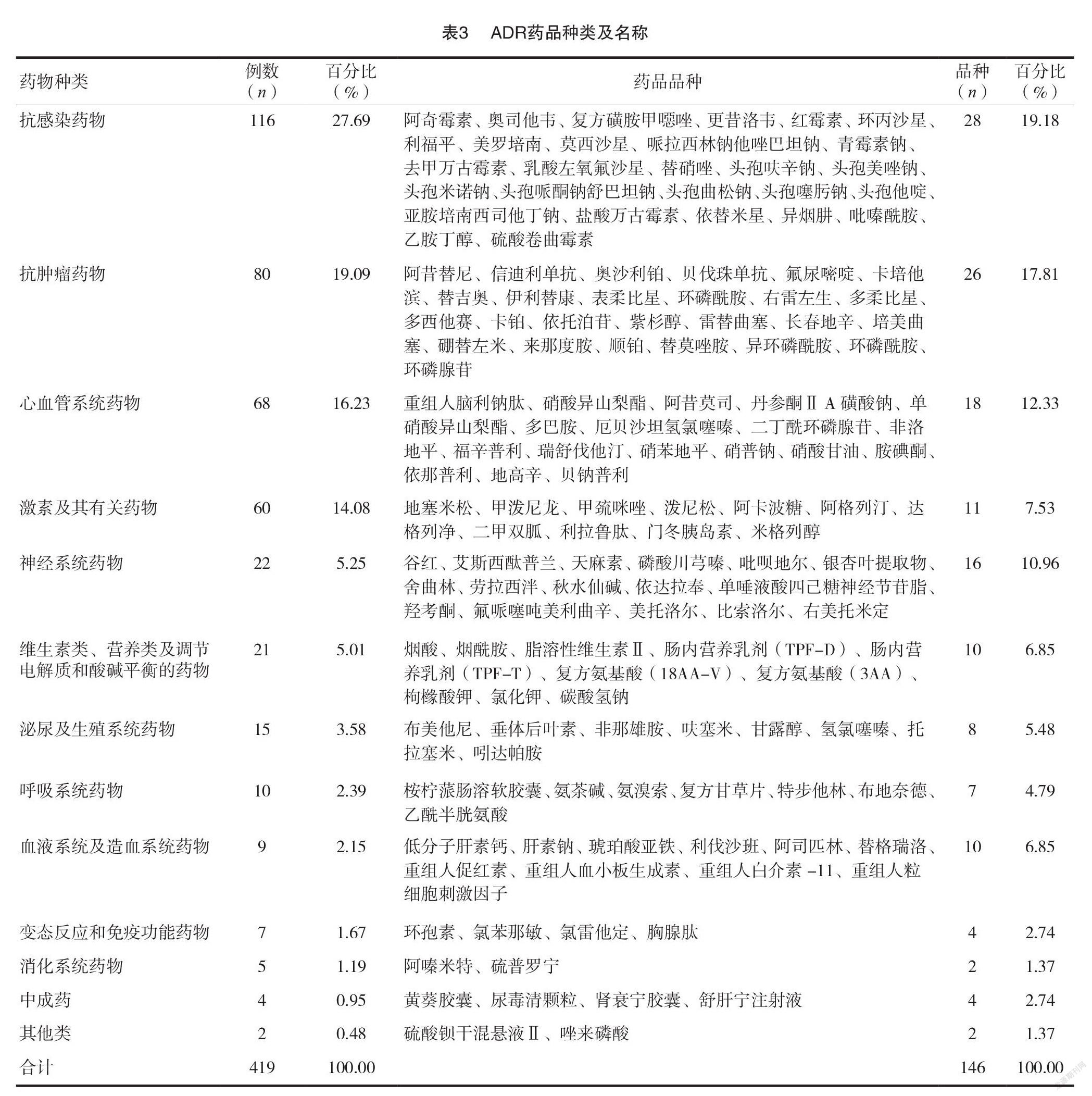
[摘要]目的探討錫林郭勒盟中心醫(yī)院(我院)419例藥品不良反應(yīng)(ADR)的發(fā)生特點(diǎn),促進(jìn)臨床合理用藥。方法回顧性分析我院2020年度上報(bào)至國家 ADR 監(jiān)測(cè)中心的419例 ADR 報(bào)告,對(duì)患者的性別、年齡、給藥途徑、藥物類別、ADR 涉及的器官/系統(tǒng)及臨床表現(xiàn)進(jìn)行統(tǒng)計(jì)分析。結(jié)果419例 ADR 中,女性患者數(shù)量(224例)高于男性(195例);60~69歲年齡段的患者 ADR 發(fā)生率最高(111例,占26.49%);口服給藥途徑 ADR 發(fā)生比例最高(199例,占47.49%);ADR 涉及藥物品種主要為抗感染藥物(116例,占27.69%),其次為抗腫瘤藥物(80例,占19.33%);ADR 累及的器官/系統(tǒng)以消化系統(tǒng)最常見(136例,占29.82%),主要表現(xiàn)為惡心、嘔吐等胃腸道反應(yīng)。結(jié)論抗感染藥物及抗腫瘤藥物是 ADR 監(jiān)測(cè)重點(diǎn)藥物類別,老年人是 ADR 重點(diǎn)監(jiān)測(cè)人群,口服給藥是 ADR 重點(diǎn)監(jiān)測(cè)給藥途徑,惡心、嘔吐等消化道反應(yīng)是提示 ADR 發(fā)生的重要信號(hào)。
[關(guān)鍵詞]藥品不良反應(yīng);監(jiān)測(cè);用藥安全;風(fēng)險(xiǎn)評(píng)估
[中圖分類號(hào)] R95? [文獻(xiàn)標(biāo)識(shí)碼] A?? [文章編號(hào)]2095-0616(2021)24-0151-05
Analysis of ADR reports of 419 cases in a hospital
ZHOU? Wei??? MA? Tianyu??? NA? Ronghua??? DAI? Yajun
Department of Pharmacy, Central Hospital of Xilingol League, Inner Mongolia, Xilinhot 026000, China
[Abstract] Objective To explore the characteristics of adverse drug reactions (ADR) in 419 cases in Xilingol League Central Hospital (our hospital), and to promote clinical rational drug use. Methods Reports of 419 cases of ADR in our hospital in 2020 reported to the National ADR Monitoring Center were analyzed retrospectively, and the gender, age, route of administration, drug category, organs/systems involved in ADR and clinical manifestations of patients were analyzed statistically. Results Among 419 cases with ADR, the number of female patients (224 cases) was more than that of male patients (195 cases). Patients aged 60-69 years old had the highest incidence of ADR (111 cases, accounting for 26.49%). The proportion of ADR related to oral administration was the highest (199 cases, accounting for 47.49%). The main drug varieties involved in ADR were anti-infective drugs (116 cases, accounting for 27.69%), followed by anti-tumor drugs (80 cases, accounting for 19.33%). The digestive system (136 cases, accounting for 29.82%) was the most common organ/system involved in ADR, and the main manifestations were gastrointestinal reactions such as nausea and vomiting. Conclusion Anti-infective drugs and anti-tumor drugs are the key drug categories in ADR monitoring, the elderly are the key population in ADR monitoring, oral administration is the key administration route in ADR monitoring, and digestive tract reactions such as nausea and vomiting are important signals of the occurrence of ADR.
[Key words] Adverse drug reactions; Monitoring; Medication safety; Risk assessment
藥品在疾病診斷、治療和預(yù)防等領(lǐng)域?yàn)槿祟惤】祹硪嫣嶽1]。但在臨床疾病治療過程中,常因藥品不良反應(yīng)(adverse drug reaction, ADR)而影響治療效果,甚至可能對(duì)患者造成生命威脅[2]。為了解錫林郭勒盟中心醫(yī)院(我院)ADR 情況,筆者回顧性分析我院2020年度上報(bào)至國家 ADR 監(jiān)測(cè)中心的419例 ADR 情況,包括患者的一般情況、ADR 相關(guān)的給藥途徑及藥品種類、ADR 累及器官/系統(tǒng)和臨床表現(xiàn)等,以探討 ADR 發(fā)生的相關(guān)因素,了解 ADR 發(fā)生的特點(diǎn)及規(guī)律,為臨床合理、安全用藥提供參考。
1資料與方法
1.1資料來源
我院2020年度上報(bào)至國家 ADR 監(jiān)測(cè)系統(tǒng),并經(jīng)上級(jí) ADR 監(jiān)測(cè)中心審核通過的 ADR 報(bào)告。……

