應用冠狀動脈CT評估冠心病合并亞臨床甲狀腺功能減退病人的斑塊特征
李玲 陳峰 劉巖


[摘要]?目的?應用冠狀動脈CT評估冠心病合并亞臨床甲狀腺功能減退癥(SCH)病人的斑塊特征。方法收集我院2016年1月—2018年12月確診SCH并經冠狀動脈CT檢查確診為冠心病的病人46例(SCH組),并以同期甲狀腺功能正常、經冠狀動脈CT檢查確診為冠心病的病人46例作為對照(對照組)。對比分析兩組病人的基線特征和斑塊特征,其中斑塊特征包括斑塊性質及鈣化積分,斑塊性質又分為鈣化斑塊、非鈣化斑塊、混合斑塊。
結果?SCH組病人的促甲狀腺素(TSH)水平顯著高于對照組(t=3.47,P<0.05),低密度脂蛋白膽固醇(LDL-C)升高病人的比例顯著高于對照組(χ2=3.21,P<0.05),LDL-C水平也顯著高于對照組(t=3.15,P<0.05),而兩組高敏C反應蛋白(hs-CRP)水平差異無顯著性(P>0.05)。SCH組的斑塊鈣化積分明顯低于對照組(t=2.72,P<0.05);SCH組非鈣化斑塊的比例顯著高于對照組(χ2=3.11,P<0.05),而對照組混合斑塊的比例顯著高于SCH組(χ2=2.74,P<0.05)。
結論?合并SCH的冠心病病人LDL-C水平較未合并SCH病人更高,非鈣化斑塊的比例明顯升高,而混合斑塊的比例較低。
[關鍵詞]?冠狀動脈疾病;甲狀腺功能減退癥;斑塊,動脈粥樣硬化;體層攝影術,X線計算機
[中圖分類號]?R541.4;R581.2
[文獻標志碼]?A
[文章編號]??2096-5532(2019)06-0631-04
doi:10.11712/jms201906001
EVALUATION OF PLAQUE CHARACTERISTICS IN PATIENTS WITH CORONARY HEART DISEASE COMPLICATED BY SUBCLINICAL HYPOTHYROIDISM BY CORONARY COMPUTED TOMOGRAPHY
LI Ling, CHEN Feng, LIU Yan
(Department of Endocrinology, the 971th Hospital of the PLA Navy, Qingdao 266071, China)
[ABSTRACT] Objective To investigate the plaque characteristics in patients with coronary heart disease complicated by subclinical hypothyroidism (SCH) by coronary computed tomography (CT).
Methods A total of 46 patients who were diagnosed with SCH and coronary heart disease by coronary CT in our hospital from January 2016 to December 2018 were enrolled as SCH group, and 46 patients with normal thyroid function who were diagnosed with coronary heart disease by coronary CT during the same period of time were enrolled as control group. Baseline characteristics and plaque characteristics were compared between the two groups. Plaque characteristics included plaque properties and calcification score, and plaque properties were classified as calcified plaques, non-calcified plaques, and mixed plaques.
Results Compared with the control group, the SCH group had significantly higher level of thyroid stimulating hormone (t=3.47,P<0.05), proportion of patients with increased low-density lipoprotein cholesterol (LDL-C) (χ2=3.21,P<0.05), and level of LDL-C (t=3.15,P<0.05), and there was no significant difference in the level of high-sensitivity C-reactive protein between the two groups (P>0.05). Compared with the control group, the SCH group had a significantly lower calcification score (t=2.72,P<0.05) and a significantly higher proportion of patients with non-calcified plaques (χ2=3.11,P<0.05), and the control group had a significantly higher proportion of patients with mixed plaques than the SCH group (χ2=2.74,P<0.05).
Conclusion Compared with the patients with coronary heart disease and without SCH, the patients with coronary heart disease and SCH have a higher level of LDL-C, a higher proportion of patients with non-calcified plaques, and a lower proportion of patients with mixed plaques.
[KEY WORDS] coronary artery disease; hypothyroidism; plaque, atherosclerotic; tomography, X-ray computed
冠心病的經典危險因素包括血脂異常、高血壓、糖尿病、吸煙等,冠心病的一級和二級預防主要是針對這些經典危險因素進行干預。但是接受標準的一級或二級預防的人群仍有心血管事件的發生,臨床表現為心絞痛、心肌梗死等,因此有必要查找經典危險因素以外的冠心病危險因素并明確其特征。甲狀腺素是一種重要的循環激素,可維持心血管穩態。促甲狀腺素(TSH)升高導致的亞臨床甲狀腺功能減退癥(SCH)與冠心病之間存在一定的關聯性[1]。有研究顯示,SCH病人的心血管疾病發病率和死亡率增加20%~80%[2-3],表明合并SCH的冠心病病人的預后較差。作為一種非傳統的心血管疾病風險因素,SCH的作用常被低估,并且缺少相關的臨床研究。斑塊特征與冠狀動脈事件是密切相關的,但目前對于冠心病合并SCH病人的斑塊特征尚不清楚。冠狀動脈CT可以精確評估冠狀動脈狹窄程度,并具有無創、準確、簡便的優勢,可用于冠心病斑塊特征的評估。本研究首次應用冠狀動脈CT評估合并SCH的冠心病病人的冠狀動脈斑塊特征。現將結果報告如下。
1?對象與方法
1.1?研究對象
2016年1月—2018年12月,收集在我院診治SCH并經冠狀動脈CT檢查確診為冠心病的病人46例(SCH組)。SCH的診斷符合第8版《內科學》的診斷標準。冠心病的診斷標準為冠狀動脈CT確定至少1支血管≥50%狹窄。該組病人均未使用甲狀腺素替代治療。按照1∶1配比,選擇同期甲狀腺功能正常、經冠狀動脈CT檢查確診為冠心病的病人46例作為對照組。本研究得到了我院倫理委員會的批準,所有病人均簽署了知情同意書。
1.2?研究方法
對比分析兩組病人的基線特征和斑塊特征,其中斑塊特征使用X射線計算機斷層成像儀(Aqui-
lion,佳能)自帶的分析軟件進行分析,包括斑塊性質及鈣化積分,斑塊性質分為鈣化斑塊、非鈣化斑塊、混合斑塊。
1.3?統計分析
采用SPSS 19.0統計軟件進行分析。計量資料以±s表示,組間比較采用t檢驗;計數資料組間比較采用χ2檢驗或Fisher精確性檢驗。以P<0.05為差異有統計學意義。
2?結?果
2.1?兩組基線特征比較
SCH組病人的TSH水平顯著高于對照組(t=3.47,P<0.05),低密度脂蛋白膽固醇(LDL-C)升高病人的比例顯著高于對照組(χ2=3.21,P<0.05),而且LDL-C水平也顯著高于對照組(t=3.15,P<0.05),兩組病人年齡、性別、糖尿病、高血壓、高敏C反應蛋白(hs-CRP)水平等冠心病危險因素差異無顯著性(P>0.05)。見表1。
2.2?兩組冠狀動脈斑塊特征比較
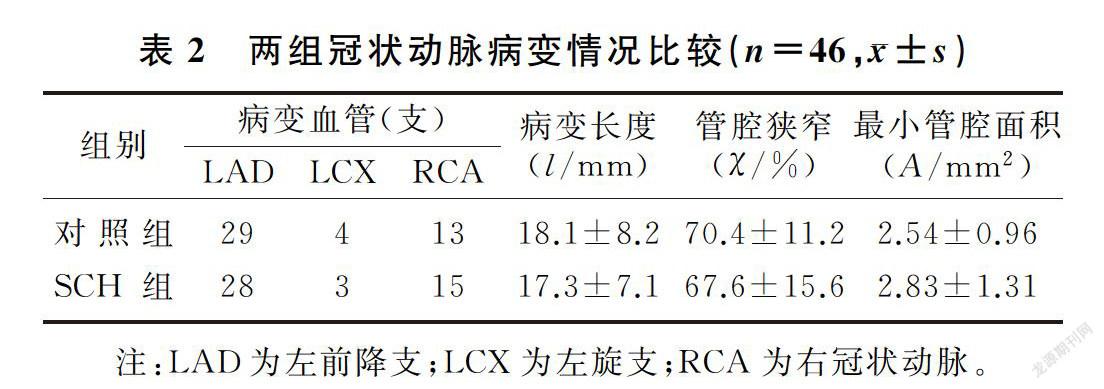
兩組病變血管均為46支,SCH組共有斑塊46個,對照組共有斑塊66個。SCH組的斑塊鈣化積分明顯低于對照組(t=2.72,P<0.05);SCH組非鈣化斑塊的比例顯著高于對照組(χ2=3.11,P<0.05),而對照組混合斑塊的比例顯著高于SCH組(χ2=2.74, P<0.05)。兩組斑塊在血管中的分布、病變長度、管腔面積和血管狹窄程度等比較差異無顯著性(P>0.05)。見表2、3。
3?討?論
近年來研究發現,甲狀腺功能減退是冠心病的又一危險因素,但目前對于冠心病合并甲狀腺功能減退病人的冠狀動脈斑塊特征并不清楚。冠狀動脈CT可準確評估冠狀動脈血管的狹窄程度、斑塊性質以及鈣化程度,具有無創、簡便的特點。故本研究應用冠狀動脈CT評估冠心病合并SCH病人的冠狀動脈斑塊特征。
本文結果表明,合并SCH的冠心病病人較未合并SCH病人有更高的LDL-C水平,非鈣化斑塊的比例明顯升高,而混合斑塊的比例較低。分析產生這種差異的原因主要為SCH導致了以脂代謝紊亂為主的代謝異常。脂質代謝異常是動脈粥樣硬化的主要原因之一,血脂升高使得血液中的脂質更易沉積于血管壁內膜,脂質與復合糖類在血管壁內膜聚集,引起血管壁內膜炎性反應,進一步導致纖維組織的增生、鈣質的沉淀,逐漸使動脈血管壁失去彈性,血管腔變得狹窄,形成動脈粥樣硬化斑塊。有研究表明,SCH病人的TC、TG、LDL-C水平明顯升高[4-8]。TSH對血脂影響的具體機制較為復雜。有文獻報道,TSH能夠促進3-羥基-3-甲基戊二酸單酰輔酶A還原酶的表達,加快膽固醇合成的速率,促進膽固醇的合成,從而增加血漿中膽固醇的濃度[9-11]。SCH發生后,ATP結合盒轉運子盒卵磷脂膽固醇酰基轉運酶的活性降低,從而導致高密度脂蛋白顆粒的合成和成熟過程受到抑制,HDL-C的水平降低。本研究未觀察到SCH組病人HDL-C水平低于對照組,可能與研究樣本量小有關[12-13]。但本文研究結果顯示,SCH組LDL-C升高病人的比例及LDL-C水平顯著高于對照組,提示冠心病合并SCH病人的斑塊形成及特征與更高的血脂水平相關。從斑塊特征來看,SCH組有更高的非鈣化斑塊比例,在冠狀動脈CT中,非鈣化斑塊代表腔內影像學中的脂質斑塊[14],這從斑塊特征方面證實了血脂升高在斑塊形成中的作用。炎癥在動脈粥樣硬化的發生和進展中起重要作用,hs-CRP是肝臟合成的一種全身性炎癥反應急性期的非特異性標志物,被認為是預測冠狀動脈斑塊不穩定的炎癥標志物[15-16]。有文獻報道,SCH病人的hs-CRP水平升高[17-19]。本研究中SCH組病人的hs-CRP水平雖然高于對照組,但差異無顯著性,可能與本研究的樣本量小有關,需要較大樣本量和前瞻性設計的研究予以證實。
[參考文獻]
[1]RODONDI N, DEN ELZEN W P, BAUER D C, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality[J].?JAMA, 2010,304(12):1365-1374.
[2]CAPPOLA A R, FRIED L P, ARNOLD A M, et al. Thyroid status, cardiovascular risk, and mortality in older adults[J].?JAMA, 2006,295(4):1033-1041.
[3]HAK A E, POLS H A, VISSER T J, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women:the Rotterdam Study[J].?Ann Intern Med, 2000,132(4):270-278.
[4]WALSH J P, BREMNER A P, BYLSARA M K, et al. Dyslipidemia dysfunction and serum lipids:a community-based study[J].?Clin Endocrinol, 2015,63(6):670-675.
[5]MARAHA R K, TANDON N, GRAG M K, et al. Dyslipidemia in subclinical hypothyroidism in an Indian population[J].?Clin Biochem, 2016,44(14/15):1214-1217.
[6]LAWAY B A, WAR F A, SHAH S, et al. Alteration of lipid parameters in patients with subclinical hypothyroidism[J]. Int J Endocrinol Metab, 2014,12(3):e17496.
[7]HUESTON W J, PEARSON W S. Subclinical hypothyroidism and the risk of hypercholesterolemia[J].?Ann Fam Med, 2014,2(4):351-355.
[8]INECK B A. Effects of subclinical hypothyrodism and its treatment on serum lipids[J].?Ann Pharmacother, 2013,37(5):725-730.
[9]ZHANG Xiujuan, SONG Yongfeng, FENG Mei, et al. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver[J].?J Lipid Res, 2015,56(5):963-971.
[10]AHN C H, CHOI S H. New drugs for treating dyslipidemia:beyond statins[J].?Diabetes Metab J, 2015,39(2):87-94.
[11]JABBAR A, PINGITORE A, PEARCE S H, et al. Thyroid hormone and cardiovascular disease[J].?Nat Rev Cardiol, 2017,14:39-55.
[12]BOONE L R, LAGOR W R, MOYA MDA L, et al. Thyroid hormone enhances the ability of serum?to accept cellular cholesterol via the ABCA1 transporter[J].?Atherosclerosis, 2011,218(1):77-82.
[13]MIKHAIL G S, ALSHAMMARI S M, ALENEZI M Y, et al. Increased atherogenic low-density lipoprotein cholesterol in untreated subclinical hypothyroidism[J].?Endocr Pract, 2014,14(5):570-575.
[14]VIRMANI R, BURKE A P, FARB A, et al. Pathology of the vulnerable plaque[J].?J Am Coll Cardiol, 2006,47(8 Suppl):C13-C18.
[15]ITHIKI T. Thyroid hormone and vascular remodeling[J].?J Atheroscler Thromb,?2016,23(3):266-275.
[16]JAYASINGH IA, PUTHURAN P. Subclinical hypothyroidia and the risk of hypercholesterolemia[J].?J Family Med Prism Care, 2016,5(5):809-816.
[17]CHRIST-CRAIN M, MEIER C, Guglielmetti M, et al. Elevated C-reactive protein and homocysteine values:cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial[J].?Atherosclerosis, 2003,166(2):379-386.
[18]RIDKER P M. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk:moving an inflammatory hypothesis toward consensus[J].?J Am Coll Cardiol, 2007,49(21):2129-2138.
[19]TSIMIKAS S, WILLERSON J T, RIDKER P M. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients[J].?J Am Coll Cardiol, 2006,47(8 Suppl):C19-C31.

