血漿同型半胱氨酸及白細胞水平與終末期腎病患者合并主動脈夾層的相關性研究
謝家和 謝龍 胡育華
[摘要]目的 探討血漿同型半胱氨酸及白細胞(WBC)水平與終末期腎病(ESRD)患者合并主動脈夾層(AD)的關系。方法 回顧性分析2011年1月~2018年12月在贛南醫學院第一附屬醫院與贛州市人民醫院診斷為AD的125例患者的臨床資料,其中ESRD合并AD 22例(作為ESRD夾層組),分別為腎性ESRD 16例,糖尿病ESRD 2例,高血壓ESRD 4例;腎功能正常的AD 103例(作為腎功能正常夾層組)。檢測兩組患者的血漿同型半胱氨酸(Hcy)、血肌酐(SCr)、腎小球濾過率(GFR)、WBC水平,并分析Hcy與SCr、GFR及WBC的關系。結果 ESRD夾層組患者的SCr、Hcy水平分別為(927.72±465.30)、(23.89±12.16)μmol/L,均明顯高于腎功能正常夾層組的(76.71±16.51)、(16.79±9.32)μmol/L,差異有統計學意義(P<0.05)。ESRD夾層組患者的GFR及WBC水平分別為(9.93±9.01)ml/(min·1.73 m2)、(8.05±2.94)×109/L,均明顯低于腎功能正常夾層組的(106.33±15.06)ml/(min·1.73 m2)、(10.89±3.55)×109/L,差異有統計學意義(P<0.05)。相關性分析結果顯示,Hcy與SCr成正相關(r=0.18,P<0.05),GFR與Hcy成負相關(r=-0.24,P<0.01),WBC計數與Hcy無相關性(r=0.04,P=0.62)。結論 血漿高Hcy可能參與ESRD患者AD的發生,提示降低Hcy水平可能成為預防ESRD患者發生AD的一個有效措施。
[關鍵詞]同型半胱氨酸;終末期腎病;主動脈夾層;炎癥;腎功能
[中圖分類號] R543.1 ? ? ? ? ?[文獻標識碼] A ? ? ? ? ?[文章編號] 1674-4721(2019)12(a)-0004-04
Study on the correlation between plasma homocysteine and white blood cell levels and aortic dissection in patients with end-stage renal disease
XIE Jia-he1,2 ? XIE Long3 ? HU Yu-hua4 ? LI Qing-rui4 ? LIU Yong-sheng5 ? ZHONG Yi-ming1,2 ? XIE Dong-ming1,2▲
1. Department of Cardiology, the First Affiliated Hospital of Gannan Medical University, Jiangxi Province, Ganzhou ? 341000, China; 2. Key Laboratory of Cardiovascular and Cerebrovascular Disease Prevention and Control Ministry of Education, Gannan Medical University, Jiangxi Province, Ganzhou ? 341000, China; 3. Department of Geriatrics, People′s Hospital of Ganzhou City, Jiangxi Province, Ganzhou ? 341000, China; 4. School of Graduate, Gannan Medical University, Jiangxi Province, Ganzhou ? 341000, China; 5. Ruijin People′s Hospital, Jiangxi Province, Ruijin ? 342500, China
[Abstract] Objective To explore the relationship between plasma homocysteine and white blood cell (WBC) levels and aortic dissection (AD) in patients with end-stage renal disease (ESRD). Methods The clinical data of 125 patients with AD diagnosed in the First Affiliated Hospital of Gannan Medical University and People′s Hospital of Ganzhou City from January 2011 to December 2018 were retrospectively analyzed. There were 22 cases of ESRD with AD (used as the ESRD dissection group), including 16 cases of kidney disease related to ESRD, 2 cases of diabetes mellitus related to ESRD, 4 cases of hypertension related to ESRD, and the remaining 103 cases were patients with normal renal function combined with AD (used as the normal renal function dissection group). The plasma homocysteine (Hcy), serum creatinine (SCr), glomerular filtration rate (GFR), and WBC levels were measured in both groups and the relationship between Hcy and SCr, GFR, WBC was analyzed. Results The levels of SCr and Hcy in the ESRD dissection group were (927.72±465.30) and (23.89±12.16) μmol/L, respectively, which were significantly higher than those in the normal renal function dissection group for (76.71±16.51) and (16.79±9.32) μmol/L, and the differences were statistically significant (P<0.05). The GFR and WBC levels in the ESRD dissection group were (9.93±9.01) ml/(min·1.73 m2) and (8.05±2.94)×109/L, respectively, which were significantly lower than those in the normal renal function dissection group for (106.33±15.06) ml/(min·1.73 m2), (10.89±3.55)×109/L, and the differences were statistically significant (P<0.05). Correlation analysis showed that Hcy was positively correlated with SCr (r=0.18, P<0.05), GFR was negatively correlated with Hcy (r=-0.24, P<0.01), and WBC count was not correlated with Hcy (r=0.04, P=0.62). Conclusion High plasma Hcy may be involved in the occurrence of AD in patients with ESRD, suggesting that lowering Hcy levels may be an effective measure to prevent AD in patients with ESRD.
[Key words] Plasma homocysteine; End-stage renal disease; Aortic dissection; Inflammation; Renal function
終末期腎病(end-stage renal disease,ESRD)是指腎臟結構或功能異常≥3個月,腎小球濾過率(glomerular filtration rate,GFR)<15 ml/(min·1.73 m2)。ESRD患者較腎功能正常人群并發心血管疾病風險顯著增加,已嚴重影響患者的生存及預后[1-2]。主動脈夾層(aortic dissection,AD)是指主動脈壁內膜破裂,導致假腔和/或壁內血腫的形成及相應器官血流灌注受損,嚴重者可因動脈壁的破裂導致患者急性死亡[3-4]。近年來,ESRD并發AD的患者不斷增多,但還尚不清楚其與腎功能正常AD患者之間有何異同點及其可能的機制。
高同型半胱氨酸血癥(hyperhomocysteinemia,HHcy)及血管炎癥是心血管疾病發生的獨立危險因素[5-7]。相關研究表明,ESRD患者血漿同型半胱氨酸(Hcy)及炎癥水平顯著增高[8-9]。但Hcy是否參與ESRD患者合并AD的發生,目前不清楚。本研究通過比較ESRD合并AD患者與腎功能正常的AD患者之間血漿Hcy及白細胞(WBC)等指標水平差異,以期為認識ESRD合并AD的發病機制及改善患者預后提供新的依據,現報道如下。
1資料與方法
1.1一般資料
回顧性分析2011年1月~2018年12月在贛南醫學院第一附屬醫院與贛州市人民醫院經影像學明確診斷為AD的125例患者的臨床資料。AD定義及診斷標準:AD指主動脈內膜破裂,血液從內膜撕裂處進入主動脈中膜,使內膜與中膜分離,形成主動脈真假腔,其診斷主要通過主動脈增強CT或磁共振等影像學技術;ESRD的定義及診斷標準:腎功能結構或功能異常≥3個月,GFR≤15 ml/(min·1.73 m2)。排除標準:服用維生素、葉酸患者;合并惡性腫瘤、甲狀腺功能減退患者;合并馬方(Marfan)綜合征等先天性主動脈血管病變患者。其中ESRD合并AD 22例(作為ESRD夾層組),分別為腎性ESRD 16例,糖尿病ESRD 2例,高血壓ESRD 4例;腎功能正常的AD 103例(作為腎功能正常夾層組)。兩組患者的年齡、性別、收縮壓、舒張壓、心率、血小板、D-二聚體、肌酸激酶(CK)及肌酸激酶同工酶(CK-MB)等一般資料比較,差異無統計學意義(P>0.05)(表1),具有可比性。本研究經贛南醫學院第一附屬醫院醫學倫理委員會批準,所有患者對診治均知情同意并簽署了知情同意書。
1.2方法
1.2.1臨床資料數據收集 ?收集入選患者的年齡、性別、血壓(收縮壓、舒張壓)、心率、合并疾病等信息。
1.2.2血液標本收集及指標測定 ?所有患者空腹8~12 h,抽取靜脈血行血漿Hcy、血小板、D-二聚體、CK、CK-MB、血肌酐(SCr)、GFR等生化指標檢測,具體操作由贛南醫學院第一附屬醫院檢驗科完成,操作步驟參考檢測儀器及試劑盒說明書。
1.3觀察指標
比較兩組患者的Hcy、SCr、WBC、GFR指標水平,并對各指標之間的相關性進行分析。
1.4統計學方法
采用SPSS 20.0及GraphPad Prism 5.0軟件統計學軟件進行數據分析,符合正態分布的計量資料采用均數±標準差(x±s)表示,兩組間比較采用t檢驗,不符合正態分布者轉換為正態分布后行統計學分析;計數資料用率(%)表示,組間比較采用χ2檢驗;相關性分析采用直線回歸分析,以P<0.05為差異有統計學意義。
2結果
2.1兩組患者實驗室指標水平的比較
ESRD夾層組患者的SCr、Hcy水平均明顯高于腎功能正常夾層組,差異有統計學意義(P<0.05);ESRD夾層組患者的GFR及WBC水平均明顯低于腎功能正常夾層組,差異有統計學意義(P<0.05)(表2)。
2.2相關性分析
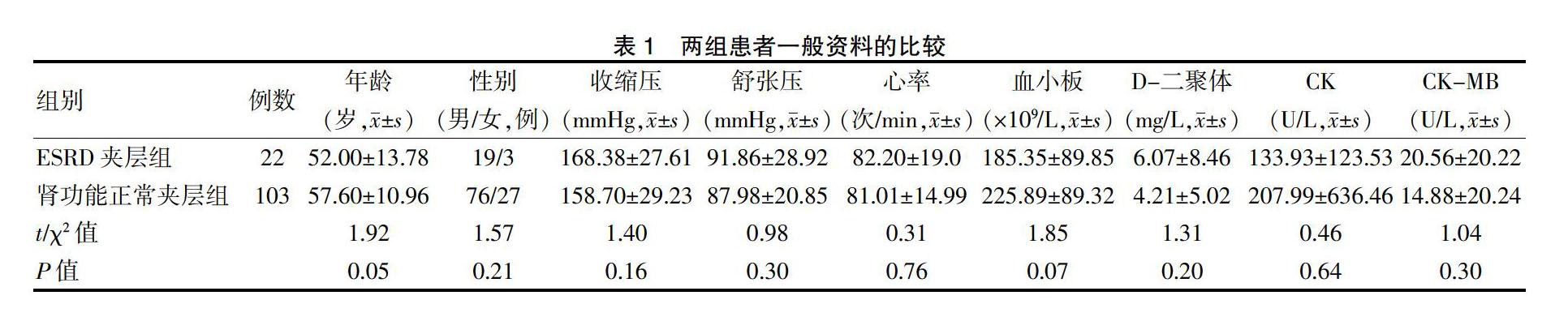
2.2.1 Hcy與SCr的相關性分析 ?患者血漿Hcy與SCr成正相關(r=0.18,F=4.40,P<0.05),直線回歸方程為Y=16.91X-0.005(圖1)。
2.2.2 Hcy與GFR的相關性分析 ?患者血漿Hcy與GFR成負相關(r=-0.24,F=7.45,P<0.01),直線回歸方程為Y=23.56X+0.06(圖2)。
2.2.3血漿WBC計數與Hcy的相關性分析
患者血漿WBC計數與Hcy無相關性(r=0.04,F=0.25,P=0.62),直線回歸方程為Y=10.68X+0.01(圖3)。
3討論
近年來,隨著人口老齡化及腎臟替代治療技術的發展,ESRD患病人數及并發心血管疾病的風險也顯著增加,已嚴重影響患者的預后,而繼發AD是其最嚴重并發癥之一[10]。目前臨床上對ESRD患者預防AD的發生除控制血壓外尚缺乏有效的措施。因此,探索ESRD患者AD的發生因素及機制對其防治具有重要意義。高Hcy是指血液中Hcy≥15 μmol/L,其在腎功能正常AD患者中的作用已有研究報道,認為高Hcy會導致AD的發生[11]。此外,HHcy是自發性顱內AD和Marfan綜合征患者繼發AD的重要危險因素[12-13]。但是目前尚不清楚Hcy在ESRD患者繼發AD中的影響。本研究結果顯示,ESRD合并AD患者的血漿Hcy水平較腎功能正常AD患者顯著升高,差異有統計學意義(P<0.05),提示高Hcy可能在ESRD患者AD的發病中發揮作用。
既往研究表明,炎癥是AD發生的重要機制,血管壁浸潤的炎癥細胞可以觸發炎癥反應及增加基質金屬蛋白酶(MMP)的分泌,從而導致彈性纖維降解及動脈壁中層結構破壞,最終促使AD的形成[14]。此外,大量的研究已證實,促炎因子可以促使AD形成,而抗炎因子可以抑制AD的擴張[15-16]。同樣,高Hcy通過促使血管炎癥反應,導致動脈粥樣硬化,其機制與Hcy激活NLRP 3炎癥小體,并介導巨噬細胞增加促炎因子白介素-1β(IL-1β)和白介素-18(IL-18)等表達有關[17]。
本研究結果顯示,ESRD合并AD患者的血漿WBC水平低于腎功能正常AD患者,差異有統計學意義(P<0.05),提示炎癥與ESRD患者AD的形成關系可能不密切。氧化應激異常是ESRD的重要病生特征,其可以損傷血管內皮細胞,破壞血管結構[1,18]。同時,活性氧自由基過多的產生也是AD形成的重要病生機制[19]。此外,有研究報道高Hcy可通過激活NADPH氧化酶加重血管損傷,導致腹主動脈瘤形成[20]。推測氧化應激等機制可能在ESRD患者AD發生中發揮重要作用,具體機制需要后續研究進一步求證。本研究結果還提示,相關性分析結果顯示,Hcy與SCr成正相關(r=0.18,P<0.05),GFR與Hcy成負相關(r=-0.24,P<0.01),WBC計數與Hcy無相關性(r=0.04,P=0.62)。
綜上所述,血漿高Hcy在ESRD患者AD發生中可能有重要作用,其機制可能與高Hcy誘發的機體氧化應激失衡等因素有密切聯系,提示降低ESRD患者的血漿Hcy水平可能成為預防包括AD在內的心血管疾病的一個有效措施。
[參考文獻]
[1]Duni A,Liakopoulos V,Rapsomanikis KP,et al.Chronic Kidney disease and disproportionally increased cardiovascular damage:Does oxidative stress explain the burden?[J].Oxid Med Cell Longev,2017,2017:9 036 450.
[2]Ronco C,Bellasi A,Di Lullo L.Cardiorenal syndrome:An overview[J].Adv Chronic Kidney Dis,2018,25(5):382-390.
[3]Melvinsdottir IH,Lund SH,Agnarsson BA,et al.The incidence and mortality of acute thoracic aortic dissection:results from a whole nation study[J].Eur J Cardiothorac Surg,2016,50(6):1111-1117.
[4]Parve S,Ziganshin BA,Elefteriades JA.Overview of the current knowledge on etiology,natural history and treatment of aortic dissection[J].J Cardiovasc Surg(Torino),2017,58(2):238-251.
[5]Leng YP,Ma YS,Li XG,et al.l-Homocysteine-induced cathepsin V mediates the vascular endothelial inflammation in hyperhomocysteinaemia[J].Br J Pharmacol,2018,175(8):1157-1172.
[6]Ganguly P,Alam SF.Role of homocysteine in the development of cardiovascular disease[J].Nutr J,2015,14:6.
[7]Holtzman JL.The role of low levels of the serum glutathione-dependent peroxidase and glutathione and high levels of serum homocysteine in the development of cardiovascular disease[J].Clin Lab,2002,48(3-4):129-130.
[8]Ferechide D,Radulescu D.Hyperhomocysteinemia in renal diseases[J].J Med Life,2009,2(1):53-59.
[9]Malecki R.Hyperhomocysteinemia as a risk factor for cardiovascular diseases in patients with chronic renal failure[J].Pol Arch Med Wewn,2000,104(4):695-701.
[10]Schiffrin EL,Lipman ML,Mann JF.Chronic kidney disease:effects on the cardiovascular system[J].Circulation,2007, 116(1):85-97.
[11]Sbarouni E,Georgiadou P,Analitis A,et al.High homocysteine and low folate concentrations in acute aortic dissection[J].Int J Cardiol,2013,168(1):463-466.
[12]Luo H,Liu B,Hu J,et al.Hyperhomocysteinemia and methylenetetrahydrofolate reductase polymorphism in cervical artery dissection:a meta-analysis[J].Cerebrovasc Dis,2014,37(5):313-322.
[13]Jiménez-Altayó F,Meirelles T,Crosas-Molist E,et al.Redox stress in Marfan syndrome: Dissecting the role of the NADPH oxidase NOX4 in aortic aneurysm[J].Free Radic Biol Med,2018,118:44-58.
[14]Nienaber CA,Clough RE,Sakalihasan N,et al.Aortic dissection[J].Nat Rev Dis Primers,2016,2:16 053.
[15]Xu H,Du S,Fang B,et al.VSMC-specific EP4 deletion exacerbates angiotensin Ⅱ-induced aortic dissection by increasing vascular inflammation and blood pressure[J].Proc Natl Acad Sci U S A,2019,116(17):8457-8462.
[16]Yoshida S,Yamamoto M,Aoki H,et al.STAT3 activation correlates with adventitial neutrophil infiltration in human aortic dissection[J].Ann Vasc Dis,2019,12(2):187-193.
[17]Wang R,Wang Y,Mu N,et al.Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice[J].Lab Invest,2017,97(8):922-934.
[18]Duni A,Liakopoulos V,Roumeliotis S,et al.Oxidative stress in the pathogenesis and evolution of chronic kidney disease:untangling Ariadne′s thread[J].Int J Mol Sci,2019,20(15):E3711.
[19]Timkova V,Tatarkova Z,Lehotsky J,et al.Effects of mild hyperhomocysteinemia on electron transport chain complexes,oxidative stress,and protein expression in rat cardiac mitochondria[J].Mol Cell Biochem,2016,411(1-2):261-270.
[20]Liu Z,Luo H,Zhang L,et al.Hyperhomocysteinemia exaggerates adventitial inflammation and angiotensin II-induced abdominal aortic aneurysm in mice[J].Circ Res,2012,111(10):1261-1273.
(收稿日期:2019-08-06 ?本文編輯:任秀蘭)

