一種改良大鼠骨髓間充質干細胞培養方法
王博榮 魯曦 張敏龍 李聰聰 李鵬程 王雅寧 金發光
·論著·
一種改良大鼠骨髓間充質干細胞培養方法
王博榮1魯曦1張敏龍1李聰聰1李鵬程1王雅寧2金發光1
目的建立大鼠骨髓間充質干細胞(BMSCs)的分離、改良培養、純化方法,并進行細胞形態學觀察、表面標志物鑒定及多向分化能力檢測。方法通過改良全骨髓貼壁法對4周齡SD雄性大鼠脫頸處死,無菌條件下分離出骨髓進行原代培養、消化傳代培養及純化。對BMSCs進行形態學觀察,收獲第四代BMSCs進行流式細胞儀檢測其細胞表面標記物CD90、CD29、CD34、CD45的表達率及向成脂方向誘導分化。結果BMSCs的原代培養形態學觀察可見骨髓細胞接種于培養皿后,細胞呈圓型,大小不一,懸浮于培養液中。24 h后部分細胞開始貼壁,呈圓形、梭形或多角形。通過換液去除未貼壁的雜質細胞,可見短梭形、星形細胞分散貼壁生長,四五天可見放射狀排列的細胞集落,伸出長短不一、粗細不均的突起,梭形細胞為主,胞漿豐富,胞核大、核仁清晰。7~8 d細胞呈集落生長,融合80%~90%,呈漩渦狀,同向排列,9~10 d細胞排列緊密,逐漸融合成片。傳代培養可見消化傳代后,傳代細胞24 h完全貼壁生長。細胞形態均一,呈梭形生長,細胞生長旺盛。四至五天可傳代1次。可穩定連續傳代7代以上,細胞形態及生長速度未見明顯變化。BMSCs表面標記物的表達通過流式細胞儀檢測結果顯示,培養的第4代大鼠BMSCs均一表達CD90,CD29,陽性率分別為96.9%,96.6%;而CD34,CD45,呈陰性,陽性率分別為0.395%,7.56%。BMSCs加入成脂誘導劑后18 d,誘導而成的脂肪細胞累積脂質,脂滴變大,合并呈串珠狀,經油紅O染色呈鮮紅色。結論與傳統全骨髓貼壁法相比,改良后的全骨髓貼壁法操作步驟簡單,降低離心對細胞的損害,減少了污染機會,節省經費,且分離的BMSCs細胞活性高,可大量分離、純化、擴增,所獲細胞具有間充質干細胞的一般生物學特性,經誘導培養后具有多向分化潛能。可為組織器官缺損性疾病、惡性腫瘤等的治療和組織工程提供充足的種子細胞來源,具有重要的現實意義。
骨髓間充質干細胞; 原代培養; 形態學; 分化; 鑒定
骨髓間充質干細胞(bone marrow mesenchymal stem cells, BMSCS)是具有自我復制和多向分化潛能的非造血干細胞[1]。目前用于分離BMSCS的方法主要有4種:全骨髓貼壁法、密度梯度離心法、細胞表面分子標記分選法、細胞篩選法,其中以全骨髓貼壁法和密度梯度離心法最為常用。后兩種方法雖然分離出的細胞純度高,但由于分離細胞數量少,活性低,加之技術難、成本高,因此較少采用。目前還沒有單一某種特異性的方法用來鑒定BMSCS,因此本實驗采用形態學觀察初步判斷BMSCS,然后通過表面標志物的表達以及在誘導條件下對其多向分化能力的判定,逆向證明體外分離培養的細胞是否為BMSCs,以確立高效穩定骨髓的大鼠BMSCS分離培養體系和鑒定方案。體外分離出培養純度高、活力強、生物特性均一的BMSCs對組織工程及細胞的體內、體外實驗顯得至關重要。本實驗通過BMSCs的黏附特性,應用全骨髓貼壁培養法,并改良優化,提取過程中無需離心細胞,并將其胎牛血清濃度提高到15%,建立了一個簡便、有效的原代培養、增殖和純化BMSCs的方法,旨在觀察大鼠BMSCs的生物學特性及其成脂分化等多種分化潛能,為組織工程尋找良好的種子細胞提供實踐。
材料與方法
一、主要材料
SD雄性大鼠購自第四軍醫大學,SPF級,實驗過程中對動物的處置符合中華人民共和國科學技術部2006年頒布的《關于善待實驗動物的指導性意見》標準[2]。L-DMEM培養液、胎牛血清購自Gibco公司, 油紅O染色試劑、胰蛋白酶、青鏈霉素混合液購自Sigma公司,成脂細胞誘導液購自賽業公司,CD34、CD29、CD45、CD90表面分子抗體購自B公司,CD34、CD29、CD45、CD90表面分子抗體同型對照購自abcom公司。主要儀器設備: CO2恒溫培養箱購自力康公司,超凈工作臺購自蘇凈安泰,FACS Calibur 流式細胞儀購自BD公司。
二、研究方法
1. BMSCs的原代分離培養:采用全骨髓貼壁改良法:4周齡SD雄性大鼠脫頸處死,70%乙醇全身浸泡消毒10 min。無菌條件取股骨及脛骨,去除骨上附著軟組織,放置在 PBS緩沖液皿中。剪掉股骨和脛骨的骨骺端,露出骨髓腔,用5 ml注射器抽取添加青、鏈霉素、15%胎牛血清L-DMEM培養基,沖出骨髓在DMEM培養皿中,直至骨髓腔呈白色。無需離心,將盛有骨髓條的培養皿置于37 ℃、含有5% CO2飽和濕度培養箱中培養。
2. BMSCs的培養、純化及傳代:原代培養過程中,接種后72 h全量更換培養基,可將其中未貼壁細胞去除,以后每3 d全量更換新鮮培養基。待細胞鋪滿培養瓶底至細胞融合成單層,密度長至70%~80%融合時,用0.02%EDTA+0.25%胰酶消化,1︰2的比例進行傳代培養。
3. BMSCs的形態學觀察:培養后每日用倒置相差顯微鏡觀察細胞形態變化及生長狀況并拍照。
4. BMSCs鑒定:流式細胞儀檢測細胞表面標記[3]:收獲P4代生長狀態良好細胞,0.02%EDTA+0.25%胰酶消化,吹打混勻,分別分成三管,A空白對照組,B抗體組,C同型對照組,計數細胞,各管細胞密度為1×106,4 ℃,300×g 離心,5 min,用PBS 500 μl漂洗細胞1次,去上清,加50 μl PBS,B管依次加入單克隆抗體CD34、CD45、CD90、CD29各2 μl。C管依次加入同型陰性對照。避光冰上孵育30 min,用PBS 2 ml洗滌細胞1次,以除去未結合抗體,以離心半徑15 000 r/min離心5 min去上清,再重懸細胞,過濾,流式細胞儀進行檢測分析。
5. BMSCs體外定向誘導分化[4-5]:選擇第4代BMSCs,按4×103個/cm2濃度接種于6孔細胞培養板,待細胞貼壁生長至細胞密度達80%時,在各誘導孔的完全培養液中加入成脂細胞誘導劑(10 mmol/L地塞米松、10 mg/L胰島素)。各誘導孔隔天換液1次,以未加誘導培養液的細胞培養孔作為對照。成脂細胞誘導劑誘導18 d后,經40 g/L多聚甲醛固定,對誘導分化的脂肪細胞進行油紅O染色。
結 果
一、BMSCs的形態學觀察
原代培養:骨髓細胞接種于培養皿后,細胞呈圓型,大小不一,懸浮于培養液中。24 h后部分細胞開始貼壁,呈圓形、梭形或多角形。通過換液去除未貼壁的雜質細胞,可見短梭形、星形細胞分散貼壁生長,4~5 d可見放射狀排列的細胞集落,伸出長短不一、粗細不均的突起,梭形細胞為主,胞漿豐富,胞核大、核仁清晰。7~8 d細胞呈集落生長,融合80%~90%,呈漩渦狀,同向排列,9~10 d細胞排列緊密,逐漸融合成片。傳代培養:消化傳代后,傳代細胞24 h完全貼壁生長。細胞形態均一,呈梭形生長,細胞生長旺盛。四五天可傳代1次。可穩定連續傳代7代以上,細胞形態及生長速度未見明顯變化,見圖1、2。
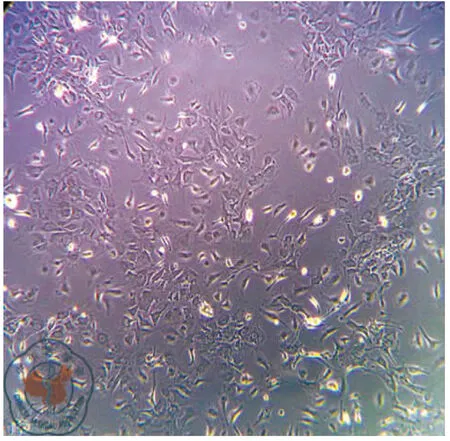
圖1 骨髓間充質干細胞形態學觀察(×20)
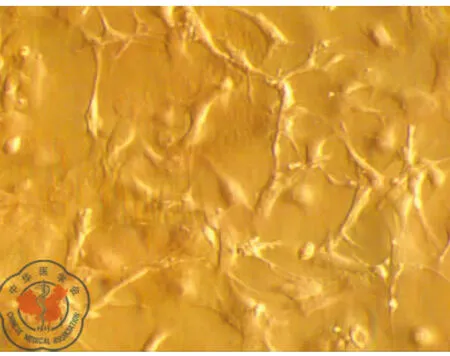
圖2 骨髓間充質干細胞形態學觀察(×200)
二、BMSCs表面記物的表達
流式細胞儀檢測結果顯示,培養的第4代大鼠BMSCs均一表達CD90, CD29,陽性率分別為96.9%, 96.6%;而CD34, CD45,呈陰性,陽性率分別為0.395%, 7.56%,見圖3。
三、BMSCs成脂誘導分化鑒定
BMSCs加入成脂誘導劑后18 d,誘導而成的脂肪細胞累積脂質,脂滴變大,合并呈串珠狀。經油紅O染色呈鮮紅色,見圖4。
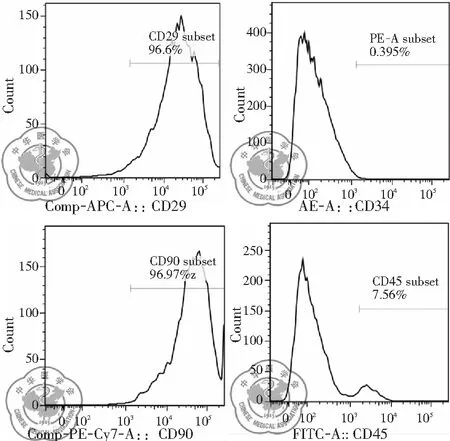
圖3 大鼠骨髓間充質干細胞表面標志物的表達
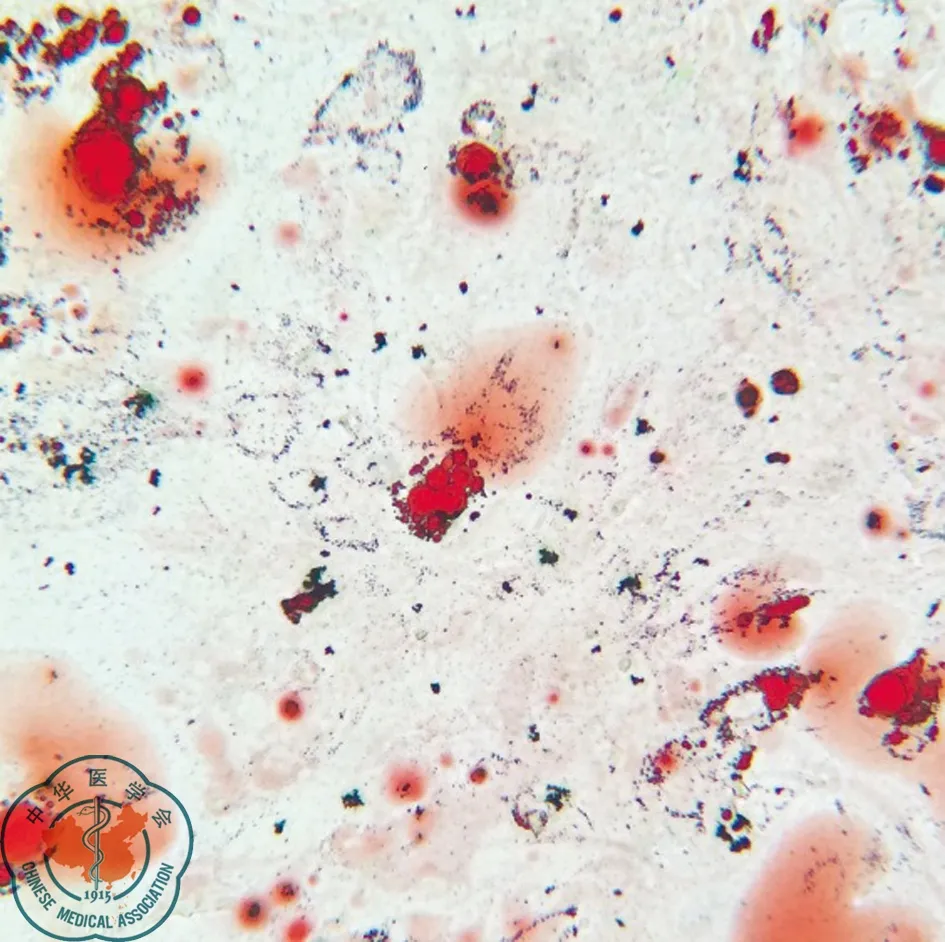
圖4 大鼠骨髓間充質干細胞成脂誘導18 d油紅O染色(×200)
討 論
BMSCs是存在于骨髓中具有高度自我更新能力和多向分化潛能的成體干細胞。1966年,Friedenstein等[6]首次對BMSCs進行了描述,并從大鼠骨髓細胞中分離培養了骨/軟骨形成祖細胞。目前,雖然已能在一些組織,包括肝臟、胚胎血、臍帶血和羊水中分離培養,但是細胞的獲得和研究主要集中在骨髓組織[7-9]。
在骨髓中含有較高的BMSCs,但改變了BMSCs生長的微環境,會影響細胞活力。本實驗將全骨髓貼壁法進行改良,發現原代培養時采用全骨髓貼壁法所分離的BMSCs無需離心,72 h首次換液細胞貼壁數量多,之后同期觀察其貼壁細胞數量明顯優于離心后干細胞組貼壁。此外,全骨髓貼壁改良優化后所分離的細胞活性高,增殖能力強,原代細胞首次融合僅需5~7 d;其經流式細胞術純化鑒定,其純度達標,不亞于傳統骨髓貼壁法經離心后所獲得的細胞純度,并避免了貼壁細胞數量相對少,細胞生長相對緩慢,活性相對低,及經離心后其原代細胞首次融合需10 d以上等缺點。這可能是因為分離出的細胞不經過離心環節,可以減少其污染概率,及離心造成的損傷,并且利用BMSCs的較強貼壁性對其進行分離,而細胞的生存、生長需要的某些因子來自于與其伴生的其他細胞的緣故。該法在很大程度上模擬了體內BMSCs的生長環境,含有的若干生長因子及促黏附物質促進了BMSCs的貼壁生長。原始的骨髓貼壁法經離心后BMSCs中可能失去了促進BMSCs貼壁的一些生長因子及促黏附物質,喪失了骨髓中原有的微環境,因此傳統方法所分離的BMSCs生長速度較改良優化法要慢。BMSCs改良優化法在傳代后純度并沒有太大區別,特別是在三四代以后。光鏡下觀察大鼠BMSCs,呈成纖維狀或紡錘狀,貼壁生長,增殖快,細胞相互緊密貼附生長,逐漸融合成片,沿胞體長軸有序排列,呈旋渦狀。BMSCs經傳代純化,第4代骨BMSCs形態單一均勻,融合后呈典型的極性,漩渦狀生長。目前,國際細胞治療學會間充質及組織干細胞委員會提出的鑒定人來源BMSCs的3條最低標準[10]是:①對塑料底物的貼附特性;②CD105、CD29及CD90等陽性表達率≥95%,而CD45、CD34、CD14或CD11b、CD79a或CD19、HLA-DR等的陽性表達率≤5%;③具有多向分化潛能。因此,實驗選取BMSCs表達陽性的指標CD29、CD90,以及表達陰性的指標CD34和CD45作為鑒定參考指標[11-20]。流式細胞儀檢測結果顯示,第4代BMSCs不表達造血前體細胞標志抗原CD34和白細胞標志抗原CD45,表達整合素家族成員的CD29、CD90。實驗結果顯示在成脂誘導條件下,第4代BMSCs分別表現出成脂肪細胞表型特征,說明體外分離培養的細胞為BMSCs。
以上結果表明,與傳統全骨髓貼壁法相比,改良后的全骨髓貼壁法操作步驟簡單,既降低了離心對細胞的損害,又減少了污染機會,節省經費,且分離的BMSCs貼壁時間短,增殖快,細胞數量多,經傳代后能夠純化,提示全骨髓貼壁改良優化方法是一種更加簡單有效的BMSCs分離方法。BMSCs分離方法可獲得高純度、增殖能力強、數量足的BMSCs,可為組織器官缺損性疾病、惡性腫瘤等的治療和組織工程提供充足的種子細胞來源,具有重要的現實意義。
1 Gao Y, Zhu Z, Zhao Y, et al. Multiline age potential research of bovine amniotic fluid mesenchymal stem cells[J]. Int J Mol Sci, 2014,15(3): 3698-3710.
2 中華人民共和國科學技術部. 關于善待實驗動物的指導性意見. 2006-09-30.
3 Bühring HJ, Treml S, Cerabona F, et al. Phenotypic characterization of distinct human bone marrow-derived MSC subsets[J]. Ann NY Acad Sci, 2009, 1176: 124-134.
4 常穎, 齊欣,卜麗莎. 成人骨髓間充質干細胞體外多向分化潛能特性的研究[J]. 中國危重病急救醫學, 2005, 17(2): 95-97.
5 賈秀娟, 孫曉娟, 徐麗麗. 特定微環境條件下人骨髓間充質干細胞定向誘導分化為脂肪細胞[J]. 中國組織工程研究與臨床康復, 2008, 12(34): 6635-6638.
6 Fridenshteǐn AIa, Piatetski -Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells[J]. Arkh Anat Gistol Embriol, 1969, 56(3): 3-11.
7 Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow[J]. Blood, 2001, 98(8): 2396-2402.
8 Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood[J]. Blood, 2004, 103(5): 1669-1675.
9 Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow[J]. Nature, 2002, 418(6893): 41-49.
10 De Ugarte DA, Alfonso Z, Zuk PA, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow[J]. Immunol Lett, 2003, 89(2-3): 267-270.
11 Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement[J]. Cytotherapy, 2006, 8(4): 315-317.
12 Fernández Vallone VB, Romaniuk MA, Choi H, et al. Mesenchymal stem cells and their use in therapy: what has been achieved?[J]. Differentiation, 2013, 85(1-2): 1-10.
13 De Schauwer C, Meyer E, Van de Walle GR, et al. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity[J]. Theriogenology, 2011, 75(8): 1431-1443.
14 Zemel′ko VI, Grinchuk TM, Domnina AP, et al. [Multipotent mesenchymal stem cells of desquamated endometrium: isolation, characterization and use as feeder layer for maintenance of human embryonic stem cell lines][J]. Tsitologiia, 2011, 53(12): 919-929.
15 Zhang N, Dietrich MA, Lopez MJ. Canine intra-articular multipotent stromal cells (MSC) from adipose tissue have the highest in vitro expansion rates, multipotentiality, and MSC immunophenotypes[J]. Vet Surg, 2013, 42(2): 137-146.
16 Sousa BR, Parreira RC, Fonseca EA, et al. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications[J]. Cytometry A, 2014, 85(1): 43-77.
17 Hwang SH, Park SH, Choi J, et al. Age-related characteristics of multipotent human nasal inferior turbinate-derived mesenchymal stem cells[J]. PLoS One, 2013, 8(9): e74330.
18 Branch MJ, Hashmani K, Dhillon P, et al. Mesenchymal stem cells in the human corneal limbal stroma[J]. Invest Ophthalmol Vis Sci, 2012 Aug 3; 53(9): 5109-5116.
19 De Cesaris V, Grolli S, Bresciani C, et al. Isolation, proliferation and characterization of endometrial canine stem cells[J]. Reprod Domest Anim, 2016, doi: 10.1111/rda.12885.
20 Phinney DG, Sensebé L. Mesenchymal stromal cells: misconceptions and evolving concepts[J]. Cytotherapy, 2013, 15(2): 140-5. doi: 10.1016/j.jcyt.2012.11.005.
(本文編輯:張大春)
王博榮,魯曦,張敏龍,等. 一種改良大鼠骨髓間充質干細胞培養方法[J/CD]. 中華肺部疾病雜志(電子版), 2017, 10(1): 25-28.
Modified culture and identification of rat bone marrow mesenchymal stem cells
WangBorong1,LuXi1,ZhangMinlong1,LiCongcong1,LiPengcheng1,WangYaning2,JinFaguang1.
1DepartmentofRespiratoryMedicine,TangduHospital,FourthMilitaryMedicalUniversity,Xi′an710038,China;2DepartmentofRespiratoryMedicine,theSecondPeople′sHospitalofBaojiCity,Baoji721000,China
JinFaguang,Email:jinfag@fmmu.edu.cn
Objective To establish the rat bone marrow mesenchymal stem cells(BMSCs) were isolated and cultured, purified and modified optimization method in vitro, to observe cell morphology, and to assess surface markers and differentiation capacity detection. Mthods The bone marrows of 4-week-old, male Sprague Dawley rats were used to obtain mMSCs. Rats were euthanized via cervical dislocation. After 72 hours, the medium was replaced and fresh medium was provided every 3 days. BMSCs were identified via flow cytometry, multi-directional differentiation capacity, and morphology. Results Primary culture: Bone marrow cells were seeded in round culture dishes of different sizes and suspended in culture medium. After 24 hours, the part of the cells adhered to the culture dish were visible as round, fusiform, or polygonal. After removal of the non-adherent cells by media replacement, the adherent cells were found to be short, spindle or star shaped, and scattered on the plastic surface. After 4 or 5 days, radially arranged cell colonies were visible, which differed in length and thickness. Spindle cells comprised the main colonies, possessing abundant cytoplasm and a large nucleus. After a week, the cells showed colony growth, were fused at approximately 80%-90%, and were reminiscent of a whirlpool, all with the same directionality. After 10 days, the cells were arranged in close proximity to one another, gradually integrating into sheet form. Culture passage: After digestion and passage, the cells adhered to the plastic surface within 24 hours. The cells were homogeneous, spindle-shaped, and displayed strong cell growth. Each passaging was performed after 4 or 5 days of growth. Cell morphology and growth rate did not significantly differ after 10 generations of stable and continuous passage. The results of flow cytometry revealed a positivity rate of CD90 expression of 96.9%, and of 96.6% for CD29. Thus, the negativity rate of CD45 was 7.56%, and that of CD34 was 0.395% in fourth generation rat mMSCs. The mMSCs were added into an adipocytes-inducing agent, where they were cultured for 18 days, during which time, lipid and lipid droplet accumulation was induced with a string of beads. The oil red O staining was bright red. Conclusions Without centrifugation of whole bone marrow adherent culture method has the advantages of simple operation, is not easy to pollute, highly active cell, bulk separation, purification and amplification of BMSCs, cells with a mesenchymal stem cell biological characteristics. After cultured with multilineage differentiation potential.
Bone marrow mesenchymal stem cells; Primary culture; Morphology; Differentiation; Identification
10.3877/cma.j.issn.1674-6902.2017.01.006
基金編號: 國家自然科學基金資助項目(81570067)
710038 西安,第四軍醫大學唐都醫院呼吸內科1721000 寶雞,寶雞市第二人民醫院呼吸內科2
金發光,Email: jinfag@fmmu.edu.cn
R563
A
2016-03-29)

