一些二茂鐵苯基亞胺化合物的合成與表征
Ikhile Monisola I Ngila J Catherine
(Department of Applied Chemistry,University of Johannesburg,Doornfontein Campus, P.O.Box 17011,Doornfontein 2028,Johannesburg,South Africa)
一些二茂鐵苯基亞胺化合物的合成與表征
Ikhile Monisola I*Ngila J Catherine*
(Department of Applied Chemistry,University of Johannesburg,Doornfontein Campus, P.O.Box 17011,Doornfontein 2028,Johannesburg,South Africa)
通過回流對應的二茂鐵苯胺和芳香醛的混合物的同樣路徑合成了一系列新穎的二茂鐵苯基亞胺化合物(5~12)。當暴露于空氣時化合物5~12穩定,不發生任何分解。所有化合物均用1H、13C NMR,MS,IR,UV-Vis和元素分析表征。還報導了化合物N-(3-bromo-2-hydroxylbenzylidene)-4-ferrocenylimine(10)的單晶結構,其結晶屬單斜晶系P21/c空間群。
二茂鐵;合成;表征;亞胺;X-射線衍射
0 Introduction
The synthesis of ferrocene based compounds has becomeanactiveresearchbecauseoftherich chemistry and properties of ferrocene[1].Over the last few years,ferrocene and its derivatives have found wide applicationinhomogeneouscatalysis[2-6],materials science[7],sensors[8-9],electroactive materials[10-12]and in medicine[13-14].In addition the easy functionalization of ferrocene and their stability in aqueous,aerobic media and their unique electronic properties is advantageous totheirwideapplications[15-17].Ornelas C.[18]had reviewed on the use of ferrocene-based compounds for medicinal applications as an active research,especially in anticancer activity.
Recently,someferrocenederivedcompounds reportedbyMathiyalaganet al.[19]havegreater antibacterial and antifungal activity than the respective standards.Various examples of enhanced activity of some drugs are reported when ferrocene fragment isincorporatedintoan organiccompound[17,20-22].A common example is tamoxifen well-known anticancer drug,when ferrocene fragment is incorporated by replacing the phenyl group of tamoxifen with ferrocene to produce a compound(Ferrocifen)that exhibits a stronger effect against breast cancer cells that are resistantto tamoxifen[18,23].Ikhile et al.[2]had also reported the synthesis and catalytic activity of some ferrocenyl based imidazolium salts.As a result of the enhanced and increased activity when ferrocene is incorporated into an organic compound,it is therefore importanttodesign,synthesizenovelferrocene derived compounds and evaluate their activity.
Imine also known as Schiff bases,are structurally nitrogen analogue of an aldehyde or ketone in which the Carbonyl group has been replaced by an imine or azomethine group[24].Schiff bases have found applications in most branches of chemistry especially in biological,inorganic and analytical chemistry[25].The biological application of Schiff bases has been recently reviewed[25].Schiff bases derived from various heterocyclic compounds have showed different ranges of biological applicationespecially asantitumor[26-27], antimicrobial[28],anti-depressant[29],anti-inflammatory[30], anti-viral[31],angiotension-II receptor antagonist[32],antioxidant[33],anti-bacterial[34],anti-glycation[35],anticonvulsant[36]and anti-tuberculosis[37].Therefore modifying the Schiff bases by incorporation of ferrocene into their molecules will improve their wide applications.
Recently,Zaheer et al.[38]synthesized some ferrocenyl Schiff bases with some low cytotoxicity and appreciable antifungal,antioxidant and DNA protection activities.Also,Chen et al.[39]synthesized three new hydroxyl substituted ferrocenyl Schiff bases from amixtureofaminoferroceneandcorresponding aldehyde in dry methanol.The three compounds synthesized by Chen et al.[39]exhibit antioxidant and anti cancer activities.The synthesis and spectroscopic characterizationofSchiffbaseligandscontaining pyridine moiety and their nickel,copper and zinc complexes have been reported[40].Therefore;itis paramounttosynthesizenewSchiffbaseswith ferrocene moiety due to the wide application of these effective compounds.Herein,we report the synthesis andcharacterizationofsomeferrocenylimine compounds,starting with either 3-ferrocenylaniline or 4-ferrocenylaniline.
1 Experimental
1.1 General procedure
All manipulations involving air and moisture sensitive compounds were performed through the use of standard Schlenk techniques under an atmosphere of dry argon.All NMR experiments were conducted on a 400 MHz Bruker Ultrashield spectrometer and samples were dissolved in deuterated chloroform. Infrared spectra were recorded with a PerkinElmer Universal ATR Spectrum 100 FTIR spectrometer.All low resolution mass data were run on the Waters Alliance3100Empower2154HPLC-mass spectrometer using Electrospray Ionisation in positive/ negative mode.Elemental analyses were performed in a Flash2000 organic elemental analyzer.UV-Visible spectra were recorded on Agilent Technologies Cary 60UV-Visiblespectrophotometer.Reagentswere purchased from Sigma-Aldrich and were used as received.The 3-ferrocenyl phenyl and 4-ferrocenyl phenylanilineweresynthesizedbyamodified literature procedures[41].Melting points were recorded on an Electrothermal,model IA8103 digital melting point apparatus and were uncorrected.
1.2 Synthesis of 3-nitrophenylferrocene and 4-nitrophenylferrocene(1 and 2)
In a two round-bottomed flasks,3-nitroaniline or 4-nitroaniline,30mLofwaterand30mLof concentrated hydrochloric acid were mixed together and cooled to 0~5℃.A solution of sodium nitrite in water was added dropwise with stirring.After the addition was complete,the solution was stirred for 30 min and kept below 5℃during this period.Ferrocene and 1 g hexadecyltrimethylammonium bromide were added to 100 mL ethyl ether and cooled to 0~5℃. The above prepared diazonium salt solution was added dropwisewithstirring.Aftertheadditionwas complete,the reaction mixture was stirred for an additional 5 h at room temperature.The reactionmixture was evaporated and the crude product was extracted with dichloromethane.
1.2.1 Synthesis of 3-nitrophenylferrocene(1)
Starting materials used were 3-nitroaniline(13.4 g,96.8 mmol),ferrocene(9.0 g,48.3 mmol)and the other starting materials as stated above(see Section 1.2).The yield was an orange powder:12.6 g,85%, m.p.112.4℃,IR(ATR,cm-1):2 922,2 853,1 522, 1 343,1 285,1 268,1 104,1 036,1 017,999,911, 894,828,807,772,742,723,673,652,615;δ H (400 MHz,CDCl3):8.26(1H,s,C6H4),8.00(1H,d,J 7.6 Hz,C6H4),7.74(1H,d,J 7.7 Hz,C6H4),7.42(1H, t,J 7.9 Hz,C6H4),4.71(2H,s,C5H4),4.40(2H,s, C5H4),4.05(5H,s,C5H5);δ C(100 MHz,CDCl3):148.62, 142.15,131.60,129.28,120.48,120.38,82.63,69.98, 66.89;m/z(ESI):307.2(M+,100%);Anal.Calcd.for C16H13NFeO2(%):C,62.57;H,4.27;N,4.56;Found (%):C,62.60;H,4.28;N,4.54.
1.2.2 Synthesis of 4-nitrophenylferrocene(2)
Starting materials used were 4-nitroaniline(14.0 g,100 mmol),ferrocene(9.0 g,48.3 mmol)and the other starting materials as stated above(see Section 1.2).The product was a violet solid:13.0 g,yield 88%,m.p.164.5℃,IR(ATR,cm-1):3 104,2 924, 2 850,2 365,1 700,1 594,1 518,1 338,1 108, 1 009,849,753,690,;δ H(400 MHz,CDCl3):8.12(2H, d,J7.6 Hz,C6H4),7.55(2H,d,J7.7 Hz,C6H4), 4.73(2H,s,C5H4),4.46(2H,s,C5H4),4.04(5H,s, C5H5);δ C(100 MHz,CDCl3):148.49,145.83,126.22, 124.12,82.03,70.88,70.33,67.51;m/z(ESI):306.9 (M+,100%);Anal.Calcd.for C16H13NFeO2(%):C, 62.57;H,4.27;N,4.56;Found(%):C,62.60;H, 4.24;N,4.57.
1.3 Synthesis of 3-ferrocenylaniline and 4-ferrocenylaniline(3 and 4)
To a stirred mixture of 3-nitrophenylferrocene,1, or 4-nitrophenylferrocene,2,in 35 mL of concentrated HCl and 50 mL ethanol was added granulated tin and the reaction mixture was heated under reflux at 50℃for 5 h.After the mixture was cooled,300 mL water was added and aqueous NaOH was added to adjust the pH value to 14 before filtration.The filtrate was extracted with DCM and dried(Na2SO4).The solvent was removed by rotary evaporation.It was then subjected to column chromatography using hexane/ diethylether(7∶3,V/V)as the eluent to give a pure compound.
1.3.1 Synthesis of 3-ferrocenylaniline(3)
Startingmaterialsusedwere3-nitrophenylferrocene(5.0 g,16.3 mmol),granulated tin(10.0 g,84.0 mmol)and the other starting materials as stated above(see Section 1.3).The product was an orange solid:3.5 g,yield 78%,m.p. 129.5℃,IR(ATR,cm-1):3 712,2 806,2 579,1 960, 1 734,1 598,1 582,1 509,1 468,1 442,1 408, 1 388,1 235,1 168,1 079,1 018,998,779,693, 648;δ H(400 MHz,CDCl3):7.07(1H,s,C6H4),6.90 (1H,d,J 7.6 Hz,C6H4),6.53(1H,t,J 7.8 Hz,C6H4), 6.51(1H,d,J 7.6 Hz,C6H4),4.58(2H,s,C5H4),4.39 (2H,s,C5H4),4.04(5H,s,C5H5);δ C(100 MHz,CDCl3): 146.25,140.32,130.25,115.17,113.12,112.95, 85.66,69.63,68.75,66.56;m/z(ESI):260.1(M+-NH2, 5%),278.2(M+,100%),278.3(M+,70%);Anal. Calcd.for C16H15NFe:C,69.34;H,5.46;N,5.05; Found:C,69.29;H,5.52;N,5.02.
1.3.2 Synthesis of 4-ferrocenylaniline(4)
Startingmaterialsusedwere4-nitrophenylferrocene(6.0 g,21.0 mmol),granulated tin(13.5 g,120.0 mmol)and the other starting materials as stated above(see Section 1.3).The product was an orange solid:4.5 g,yield 77%,m.p. 160.5℃,IR(ATR,cm-1):3 372,3 651,2 922,2 852, 1 878,1 684,1 539,1 452,1 387,1 280,1 182, 1 085,1032,998,818,727,668,634;δ H(400 MHz, CDCl3):7.27(2H,d,J 7.6 Hz,C6H4),6.76(2H,d,J 7.6 Hz,C6H4),4.54(2H,s,C5H4),4.24(2H,s,C5H4), 4.02(5H,s,C5H5),2.15(s,2H,NH2);δ C(100 MHz, CDCl3):144.89,127.12,116.19,115.17,86.76,69.44, 68.24,65.78;m/z(ESI):278.1(M+,100%),278.9(M+, 48%);Anal.Calcd.for C16H15NFe(%):C,69.34;H, 5.46;N,5.05;Found(%):C,69.33;H,5.49;N,5.07.
1.4 Synthesis of ferrocenylphenylimine(5~12)
In a pre-packed two-necked flask supplied with a magnetic stirrer was added 3-ferrocenylaniline or 4-ferrocenylaniline in 15 mL of dried ethanol,was mixedwithanequimolaramountofaromaticaldehydes in 15 mL of dried ethanol.The mixture was heated under reflux and the progress of the reaction was monitored by TLC.The required product was formed in 5~6 h.The solvent was removed under vacuum to give the crude product.
1.4.1 Synthesis of N-(3-nitro-2-hydroxylbenzylidene)-3-ferrocenylimine(5)
Starting materials used were 3-ferrocenylaniline (0.05 g,0.19 mmol)and 2-hydroxyl-3-nitrobenzaldehyde (0.03 g,0.19 mmol).The product was a brick red powder:0.075 g,yield 95%,m.p.187.9℃,IR(ATR, cm-1):3 348,2 922,2 851,2 365,1 718,1 684,1 577, 1 440,1 221,1 105,1 019,1 000,907,806,773, 727,668;δ H(400 MHz,CDCl3):8.75(1H,s,HC= N),8.10(1H,d,J 7.6 Hz,C6H3),7.99(1H,d,J 7.8 Hz,C6H3),7.85(1H,t,J 7.9 Hz,C6H3),7.82(1H,d, J 7.6 Hz,C6H4),7.14(1H,t,J 7.9 Hz,C6H4),7.09 (2H,m,C6H4),4.66(2H,s,C5H4),4.35(2H,s,C5H4), 4.03(5H,s,C5H5),3.90(1H,s,OH);δ C(100 MHz, CDCl3):160.00,130.18,126.21,126.06,120.34, 119.45,117.66,84.40,72.24,69.20;m/z(ESI):425.5 (M+,100%);Anal.Calcd.for C23H18N2O3Fe(%):C, 64.81;H,4.26;N,6.57;Found(%):C,64.79;H,4.24; N,6.54.
1.4.2 Synthesis of N-(3-bromo-2-hydroxylbenzylidene)-3-ferrocenylimine(6)
Starting materials used were 3-ferrocenylaniline (0.05 g,0.19 mmol)and 3-bromo-2-hydroxylbenzaldehyde(0.04 g,0.19 mmol).The product was a darkbrown powder:0.085 g,yield 99%,m.p.391.3℃,IR (ATR,cm-1):3 432,3 062,2 951,2 920,2 851,1 774, 1 688,1 523,1 431,1 381,1 293,1 175,1 020,1 105, 999,905,843,790,735,691,669;δ H(400 MHz, CDCl3):8.63(1H,s,HC=N),7.77(1H,s,C6H3),7.63-7.37(4H,m,C6H4),6.84(2H,s,C6H3),4.65(2H,s, C5H4),4.34(2H,s,C5H4),4.04(5H,s,C5H5);δ C(100 MHz,CDCl3):163.24,159.14,158.99,140.59,138.22, 129.97,129.34,128.21,121.59,121.22,119.27, 117.67,111.04,81.54,69.65,66.51;m/z(ESI):457.3 (M+,100%),458.1(M+,50%);Anal.Calcd.for C23H18NOBrFe(%):C,60.03;H,3.94;N,3.04;Found (%):C,60.04;H,3.95;N,3.02.
1.4.3 Synthesis of N-(3-bromo-5-chlorosalicylidene)-3-ferrocenylimine(7)
Starting materials used were 3-ferrocenylaniline (0.05 g,0.19 mmol)and 3-bromo-5-chlorosalicylaldehyde(0.04 g,0.19 mmol).The product was a reddish paste:0.07 g,yield 76%,m.p.108.6℃,IR(ATR, cm-1):3 340,2 916,2 849,2 551,2 157,1 733,1 662, 1 597,1 557,1 445,1 352,1 288,1241,1 162, 1 105,1 019,973,951,927,861,788,739,711,689, 606,587;δ H(400 MHz,CDCl3):8.55(1H,s,HC=N), 7.74(1H,s,C6H2),7.61(1H,s,C6H2),7.43(1H,d,J 7.4 Hz,C6H4),7.33(2H,s,C6H4),7.09(1H,d,J 7.5 Hz,C6H4),4.66(2H,s,C5H4),4.35(2H,s,C5H4),4.05 (5H,s,C5H5),2.03(1H,s,OH);δ C(100 MHz,CDCl3): 159.93,157.19,141.43,139.38,135.55,131.84, 129.55,129.55,125.61,123.69,119.97,119.27, 117.78,111.95,84.20,69.81,69.45,66.66;m/z(ESI): 186.7(M+-C13H8NOBrCl,91%),493.4(M+,21%);Anal. Calcd.for C23H17NOBrClFe(%):C,55.85;H,3.46;N, 2.83;Found(%):C,55.87;H,3.48;N,2.85.
1.4.4 Synthesis of N-(ferrocenylformidene)-3-ferrocenylimine(8)
Starting materials used were 3-ferrocenylaniline (0.03 g,0.11 mmol)and ferrocene carboxaldehyde (0.02 g,0.11 mmol).The product was a brown solid: 0.04g,yield 77%,m.p.329.4℃,IR(ATR,cm-1): 2 920,1 648,1 597,1 451,1 306,1 105,1 025, 1 000,817,760,692,625,580;δ H(400 MHz, CDCl3):8.36(1H,s,HC=N),7.17(1H,d,J 7.3 Hz, C6H4),7.13(1H,t,J 7.2 Hz,C6H4),6.93(1H,s,C6H4), 6.70(1H,d,J 7.2 Hz,C6H4),4.78(2H,d,J 1.8 Hz, C5H4),4.66(2H,d,J 1.8 Hz,C5H4),4.59(2H,d,J 1.7 Hz C5H4),4.30(2H,d,J 1.8 Hz,C5H4),4.26(5H,s, C5H5),4.04(5H,s,C5H5);δ C(100 MHz,CDCl3):159.50, 149.44,140.44,125.69,119.71,115.39,86.95,82.95, 70.11,69.98,69.69,69.01,67.43,66.57;m/z(ESI): 472.1(M+,50%),472.8(M+,30%);Anal.Calcd.for C27H23NFe2(%):C,68.54;H,4.90;N,2.96;Found(%): C,68.53;H,4.93;N,2.98.
1.4.5 Synthesis of N-(2-hydroxyl-3-nitrobenzylidene)-4-ferrocenylimine(9)
Starting materials used were 4-ferrocenylaniline (0.057 g,0.21 mmol)and 2-hydroxyl-3-nitrobenzaldehyde(0.034 4 g,0.21 mmol).The product was a brickred crystal:0.07 g,yield 80%,m.p.234.8℃,IR (ATR,cm-1):3 427,3 080,2 918,2 850,2 108,1 730, 1 619,1 526,1 453,1 349,1 242,1 083,955,834, 813,742,692.21;δ H(400 MHz,CDCl3):8.76(1H, s,HC=N),8.10(1H,d,J 7.4 Hz C6H3),7.84(1H,J 8.2 Hz C6H3),7.54(1H,d,J 7.3 Hz,C6H3),7.30(2H, d,J 7.7 Hz,C6H4),6.64(2H,d,J 7.8 Hz,C6H4),4.52 (2H,s,C5H4),4.29(2H,s,C5H4),4.04(5H,s,C5H5);δ C(100 MHz,CDCl3):158.96,152.34,149.26,142.56, 138.46,134.36,133.64,128.56,127.12,125.12, 115.49,84.15,69.37,68.19,65.80;m/z(ESI):425.5 (M+,100%);Anal.Calcd.for C23H18N2O3Fe(%):C, 64.81;H,4.26;N,6.57;Found(%):C,64.78;H, 4.30;N,6.53.
1.4.6 Synthesis of N-(3-bromo-2-hydroxylbenzylidene)-4-ferrocenylimine(10)
Starting materials used were 4-ferrocenylaniline (0.047 g,0.17 mmol)and 3-bromo-2-hydroxylbenzaldehyde(0.034 g,0.17 mmol).The product was a brown crystal:0.058 g,yield 73%,m.p.241.6℃,IR(ATR, cm-1):3 425,3 093,2 917,2 850,1 733,1 675,1 589, 1 521,1408,1 281,1 183,1 183,1105,888,819,771, 729,691,655;δ H(400 MHz,CDCl3):8.64(1H,s,HC= N),7.62(2H,d,J 7.5 Hz C6H4),7.51(2H,d,J 7.8 Hz, C6H4),7.37(1H,d,J 7.5 Hz,C6H3),7.34(1H,d,J 7.4 Hz C6H4),6.83(1H,d,J 7.2 Hz,C6H3),4.65(2H,s, C5H4),4.34(2H,s,C5H4),4.05(5H,s,C5H5);δ C(100 MHz,CDCl3):162.31,159.92,146.51,136.06,131.24, 129.72,126.99,122.49,121.30,119.71,118.98, 111.23,84.50,69.74,69.34,66.56;m/z(ESI):326.0 (M+-C4H3Br,100%),456.4(M+-1,67%),458.0(M+, 54%),458.1(M+,54%);Anal.Calcd.for C23H18NOBrFe (%):C,60.03;H,3.94;N,3.04;Found(%):C,59.99; H,3.91;N,3.07.
1.4.7 Synthesis of N-(3-bromo-5-chlorosalicyl)-4-ferrocenylimine(11)
Starting materials used were 4-ferrocenylaniline (0.044 g,0.16 mmol)and 3-bromo-5-chlorosalicylaldehyde(0.037 g,0.16 mmol)The product was a dark brown powder:0.06 g,yield 77%,m.p.60.1℃,IR (ATR,cm-1):3 424,3 086,2 923,2 452,1 982,1 736, 1 616,1 523,1 441,1 286,1 135,1 004,815,758, 752;δ H(400 MHz,CDCl3):8.56(1H,s,HC=N),7.61 (2H,d,J 7.2 Hz,C6H4),7.51(2H,d,J 7.6 Hz C6H4), 7.41(1H,s,C6H2),7.33(1H,s,C6H2),4.65(2H,s,C5H4), 4.37(2H,s,C5H4),4.07(5H,s,C5H5),δ C(100 MHz, CDCl3):160.36,158.17,156.74,135.00,130.30,129.99, 129.52,126.72,122.23,121.06,111.57,83.73,69.46, 69.16,66.28.;m/z(ESI):273.1(M+-C7H4BrClO,100%), 495.2(M+,12%),494.6(M+,1%);Anal.Calcd.for C23H17NOBrClFe(%):C,55.85;H,3.46;N,2.83;Found (%):C,55.83;H,3.43;N,2.80.
1.4.8 Synthesis of N-(ferrocenylformidene)-4-ferrocenylimine(12)
Starting materials used were 4-ferrocenylaniline (0.049 g,0.18 mmol)and ferrocene carboxaldehyde (0.038 g,0.18 mmol).The product was a reddish brown powder:0.059 g,yield 70%,m.p 159.1℃.IR (ATR,cm-1):3 067,2 919,2 854,2 161,1 733, 1 632,1 589,1 521,1 439,1 359,1 244,1 133, 1 033,970,846,770,727,690,655;δ H(400 MHz, CDCl3):8.38(1H,s,HC=N),7.47(2H,d,J 8.4 Hz, C6H4),7.30(2H,d,J 8.6 Hz,C6H4),4.79(2H,d,J 1.8 Hz,C5H4),4.63(2H,d,J 1.8 Hz,C5H4),4.61(2H,d,J 1.7 Hz,C5H4),4.30(2H,d,J 1.8 Hz,C5H4),4.24(5H, s,C5H5),4.04(5H,s,C5H5);δ C(100 MHz,CDCl3): 160.44,136.47,130.54,129.21,121.96,85.26,82.71, 69.69,69.64,69.44,69.36,69.17,68.93;m/z(ESI): 278.3(M+-C11H10Fe,100%),475.3(M+,40%);Anal. Calcd.for C27H23NFe2(%):C,68.54;H,4.90;N,2.96; Found(%):C,68.57;H,4.88;N,2.94.
1.5 X-raycrystalDeterminationofCompound(10)
A suitable crystal was selected and mounted on Mitegen 100 micrometer loop in NVH oil on a Bruker APEX-II CCD′diffractometer.The crystal was kept at 99.98 K during data collection.Using Olex2[42],the structure was solved with the ShelXS[43]structure solution program using direct methods and refined with the ShelXL[43]refinement package using least squares minimization.
CCDC:1010246,10.
2 Results and discussion
2.1 Synthesis and Characterization
This study involves the synthesis of a series of 3-ferrocenylimine(5~8)and 4-ferrocenylimine(9~12)which were conducted via the same route. The ferrocenylphenylimine compounds were synthesized following a modified reported method[38]. Ferrocenylaniline (3 and 4) as precursors to ferrocenylimine were prepared as shown in Scheme 1 by reduction of3-nitrophenylferrocene (1) or 4-nitrophenylferrocene (2) with tin in an acidic condition[41]. Compounds 1 and 2 were synthesized by arylation of ferrocene by a diazonium salt, method adapted from a modified procedure[44].
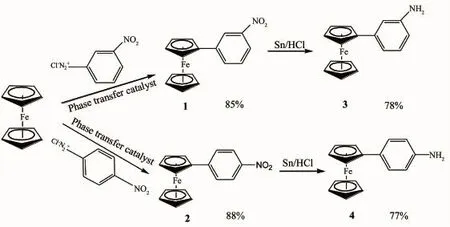
Scheme 1Synthesis of 3-ferrocenyl and 4-ferrocenyl aniline

Fig.1UV-Visible spectra for compounds 4 and 10
The target compounds(5~12)were synthesized as shown in Scheme 2 by refluxing a mixture of 3-ferrocenylaniline(3)or 4-ferrocenylaniline(4)with corresponding aromatic aldehyde.The Compounds (5~12)were obtained as air stable and in relatively high yields(70%~99%).They also showed good stabilityinsolutionwithoutdecompositionwhen exposed to air.The compounds were all characterized by1H and13C NMR spectroscopy,mass spectrometry, IR,UV-Vis spectral and elemental analysis.The imine formation for compounds 5~12 was indicated by the appearance of a strong absorption band between 1 616 and 1 688 cm-1in the IR Spectra.The C-H stretching frequencies were observed around 2 806~3 093 cm-1,also the bands that are characteristics of the presence of ferrocene in a molecule were observed around 807 and 1 183 cm-1.The NMR spectra further confirmed the formation of the ferrocenylphenylimine (compounds 5~12)by the appearance of a resonance peak at around 8.36~8.76 in1H NMR spectra attributed to the imine proton(HC=N)as compared to ferrocenylaniline(compounds 3 and 4).As expected the ferrocenyl protons were observed at around 4.02~4.71,which agree with the literature for reported related compounds[19,41,44-45].In13C NMR spectra,the imine carbon(HC=N)appeared as the most deshielded around 158.96~163.24 and the ferrocenyl moiety carbon atoms appeared at around 65.80~86.76 ppm which is in agreement with the literature[2-39].Further evidence for the formation of the compounds 1~12 was provided by the positive mode ESI-MS spectra which showed intense molecular ions corresponding to M+. The elemental analysis results are in agreement with the molecular formula of the synthesized compounds. TheUV-Visibleanalysiswascarriedoutin acetonitrile which are presented in Table 1.The compounds showed two absorption bands at about 247 and364nm.Theabsorptionspectraoftheferrocenylimines compounds 5~12 showed maxi-mum wavelengthshiftascomparedtotheferrocenyl anilines,compounds 3 and 4.The two absorption bands in compound 4(256 and 292 nm)considerably shifted about 37 and 72 nm respectively to 293 and 364 nm as compared with compound 10(Fig.1).This canbeattributedasanindicationofextended conjugated in the ferrocenylimines compounds.

Scheme 2Synthesis of 3-ferrocenylimine and 4-ferrocenylimine
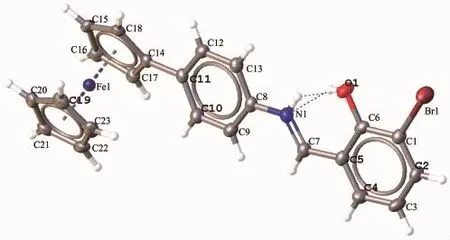
Fig.2ORTEP diagram of compound 10 shown at 50%probability thermal ellipsoids

Table 1UV-Vis Data of compounds 1~12
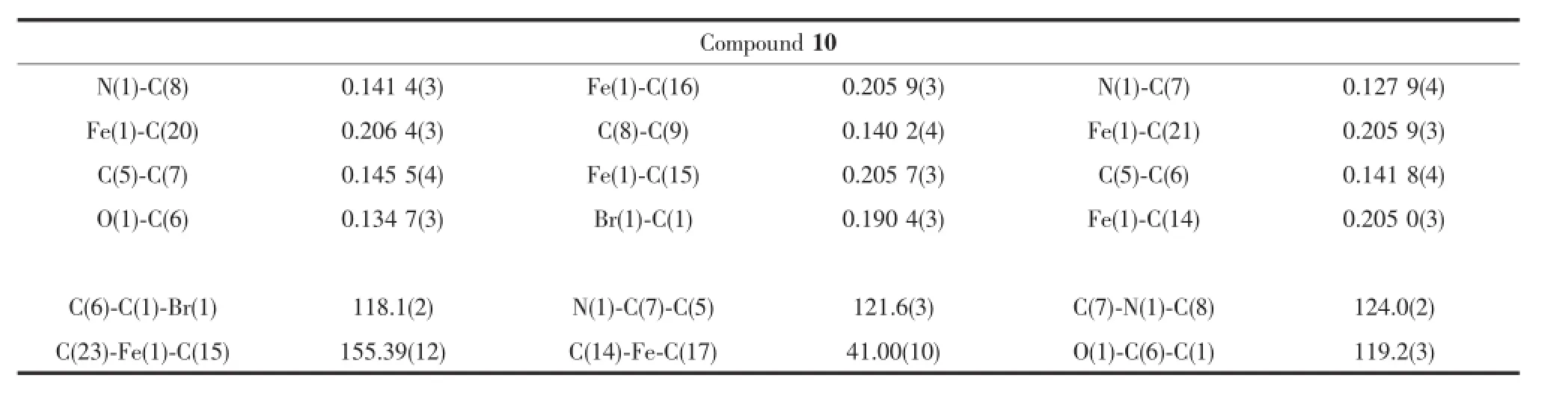
Table 2Selected bond length(nm)and angles(°)for compound 10
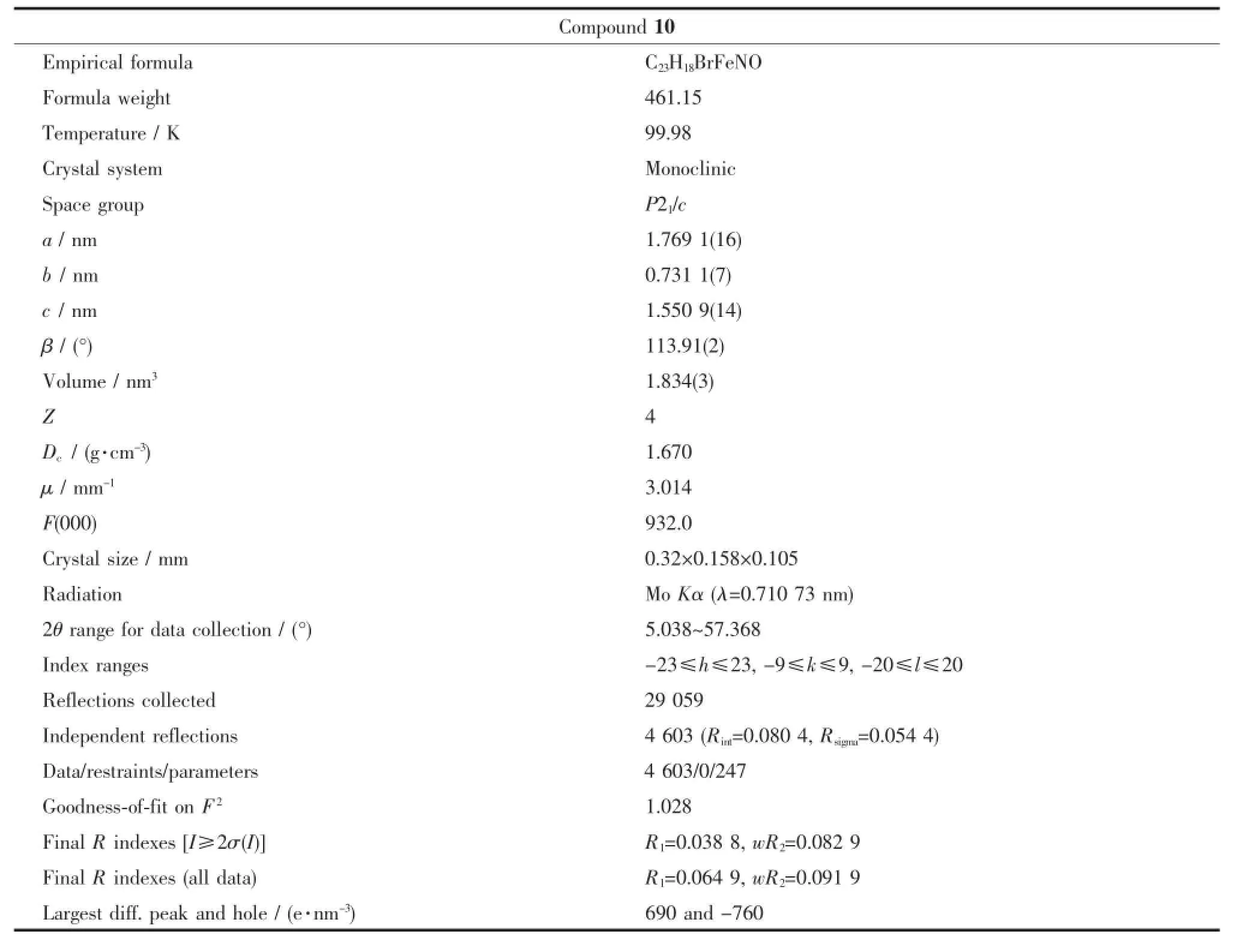
Table 3Crystal data and structure refinement for compound 10
2.2 Molecular structure of Compound 10
Crystals suitable for X-ray analysis for compound 10 was obtained by slow diffusion of hexane into saturatedsolutionofdichloromethaneatroom temperature.Compound 10 crystallized in monoclinic system with P21/c space group.The diagram is shown inFig.2.Selectedbondlengthsandanglesare presented in Table 2,while crystal data and structure refinement are summarized in Table 3.Compound 10 crystallized with one molecule in the asymmetric unit. The bond length of N1-C7(0.127 9(4)nm)is shorter than the bond length of N1-C8 which is 0.141 4(3) nm.This can be attributed to the covalent imine bond in N1-C7,thus affording a shorter bond length for it. The imine bond length N1-C7 in compound 10 also fits well with imine bond with similar structurereported[39].
3 Conclusions
A new series and air stableferrocenylimine compounds were synthesized.The molecular structure ofoneofthecompoundswasreported.The ferrocenylimine(5~12)compounds were synthesized from starting materials that are readily available, especially the ferrocenylaniline(1~4)in which their synthesis was also reported herein.
Acknowledgements:The Faculty of Science,University of Johannesburg is highly appreciated for financial support and Department of Applied Chemistry for providing facilities.The CentreforNanomaterialsScienceResearch(CNSR)is acknowledged for providing running cost of this project.Dr Charmaine Arderne(Department of Chemistry,University of Johannesburg)is highly appreciated for X-ray crystallography data collection.
[1]Coleman K S,Turberville S,Pascu S I,et al.J.Organomet. Chem.,2005,690:653-658
[2]Ikhile M I,Bala M D,Nyamori V O,et al.Appl. Organometal.Chem.,2013,27:98-108
[3]Togni A,Hayashi T(Eds.)Ferrocenes:Homogeneous Catalysis, Organic Synthesis and Material Science.Weinheim:VCH, 1995.
[4]Diallo A K,Ornelas C,Salmon L,et al.Chem.Commun., 2007:4946-4948
[5]Nazarov A A,Hartinger C.G,Arion V B,et al.Tetrahedron, 2002,58:8489
[6]Gibson V C,Gregson C K A,Halliwell C M,et al.Organomet. Chem.,2004,690:6271-6283
[7]Andrieux C P,Blocman C,Dumas-Bouchiat J M,et al.J. Am.Chem.Soc.,1980,102:3806-3813
[8]Ferreira C L,Ewart C B,Barta C A,et al.Inorg.Chem., 2006,45:8414-8422
[9]Mishra L,Dubey S K.Spectrochim.Acta A,2007,68:364-368
[10]Bildstein B,Malaun M,Kopacka H,et al.J.Organomet. Chem.,1999,572:177-187
[11]Thomas J L,Howarth J,Kennedy A M.Molecules,2002,7: 861-866
[12]Bai Y,Zhang B G,Duan C Y,et al.New J.Chem.,2006, 30:266-271
[13]Ling S,Xin Z,Yan H,et al.Chin.Chem.Lett.,2006,36: 325-330
[14]Van Staveren D R,Metzler-Nolte N.Chem.Rev.,2004,104: 5931-5985
[15]AllardyceCS,DorcierA,ScolaroC,etal.Appl. Organomet.Chem.,2005,19:1-10
[16]Neuse E W J.Inorg.Organomet.Polym.Mater.,2005,15:3 -32
[17]Fouda M F R,Abd-Elzaher M M,Abdelsamaia R A,et al. Appl.Organomet.Chem.,2007,21:613-625
[18]Ornelas C.New J.Chem.,2011,35:1973-1985
[19]Mathiyalagan K,Gopal S,Ramasamy E,et al.Int.J. ChemTech Res.,2012,4:1775-1781
[20]Kondapi A K,Satyanarayana N,Saikrishna A D.Arch. Biochem.Biophys.,2006,450:123-132
[21]Itoh T,Shirakami S,Ishida N,et al.Bioorg.Med.Chem. Lett.,2000,10:1657-1659
[22]Zhang J.Appl.Organomet.Chem.,2008,22:6-11
[23]Hillard E A,Vessieres A,Jaouen G.Top Organomet. Chem.,2010,32:81-117
[24]Mohammed I A,Subrahmanyam E V S.Acta Pharma.Sci., 2009,51:163-168
[25]Anand P,Patil V M,Sharma V K,et al.Int.J.Drug.Des. Discov.,2012,3:851-868
[26]Hranjec M,Starcevic K,Pavelic K S,et al.Eur.J.Med. Chem.,2011,46:2274-2279
[27]Chetan B,Bunha M,Jagrat M,et al.Bioorg.Med.Chem. Lett.,2010,20:3906-3910
[28]Kundariya D S,Bheshdadia B M,Joshi N K,et al.Int. J.Chem.Tech.Res.,2011,3:238-243
[29]Thomas A B,Nanda R K,Kothapalli L P,et al.Arabian J. Chem.,2011,55:960-968
[30]Sondhi S,Singh N,Kumar A,et al.Bioorg.Med.Chem., 2006,14:3758-3765
[31]Kumar K S,Ganguly S,Veerasamy R,et al.Eur.J.Med. Chem.,2010,45:5474-5479
[32]Shreenivas M T,Chetan B P,Bhat A R.J.Pharm.Sci. Tech.,2009,1:88-94
[33]Sashidhara K V,Rosaiah J N,Bhatia G,et al.Eur.J.Med. Chem.,2008,43:2592-2596
[34]Baluja S,Solanki A,Kachhadia N.J.Iranian Chem.Soc., 2006,3:312-317
[35]Khan K M,Khan M,Ali M,et al.Bioorg.Med.Chem., 2009,17:7795-7801
[36]Aly M M,Mohameda Y A,El-Bayouki K A M,et al.Eur.J. Med.Chem.,2010,45:3365-3373
[37]Bhat M A,Al-Omar M A.Acta Poloniae Pharmaceutica Drug Res.,2011,64:375-380
[38]Zaheer M,Shah A,Akhter Z,et al.Appl.Organometal. Chem.,2011,25:61-69
[39]Chen W O,Wang L,Hao Y,et al.Dalton Trans.,2013,42: 15678-15686
[40]Abd-Elzaher M M.Chin.J.Chem.Soc.,2004,51:499-504
[41]Ping H,Zhao K Q,Xu H B.Molecules,2001,6:M250 DOI: 10.3390/M250
[42]Dolomanov O V,Bourhis L J,Gildea R J,et al.J.Appl. Cryst.,2009,42:339-341
[43]Sheldrick G M.Acta Cryst.,2008,A64:112-122
[44]Ping H,Zhao K Q,Xu H B.Molecules,2001,6:M249 DOI: 10.3390/M249
[45]Motswainyana W M,Onani M O,Madieke A M.Polyhedron, 2014,41:44-51
Synthesis and Characterization of Some Ferrocenylphenylimine Compounds
Ikhile Monisola I*Ngila J Catherine*
(Department of Applied Chemistry,University of Johannesburg,Doornfontein Campus,P.O.Box 17011, Doornfontein 2028,Johannesburg,South Africa)
A novel series of ferrocenylimine compounds(5~12)were synthesized via the same route by refluxing a mixture of corresponding ferrocenylaniline with aromatic aldehyde.Compounds 5~12 were air stable without any decomposition when exposed to air.The compounds were all characterized by1H and13C NMR spectroscopy, mass spectrometry,IR,UV-Visible and elemental analysis.The single crystal structure of compound 10 was also reported,which revealed that compound 10 crystallized in monoclinic system with P21/c space group.CCDC: 1010246,10.
ferrocene;synthesis;characterization;imine;X-ray diffraction
O641.4
A
1001-4861(2015)10-2079-10
10.11862/CJIC.2015.272
2015-06-23。收修改稿日期:2015-08-31。
Supported by Faculty of Science,University of Johannesburg,South Africa.
*通訊聯系人。E-mail:jcngila@uj.ac.za,mikhile@uj.ac.za

