兩個含有混磷配體的發(fā)光銀(Ⅱ)配合物的合成和表征
張彥茹 王夢秦 崔洋哲 劉 敏 李中峰 金瓊花*,
(1首都師范大學化學系,北京100048)
(2北京工業(yè)大學材料科學與工程學院,北京100124)
兩個含有混磷配體的發(fā)光銀(Ⅱ)配合物的合成和表征
張彥茹1王夢秦1崔洋哲1劉 敏2李中峰1金瓊花*,1
(1首都師范大學化學系,北京100048)
(2北京工業(yè)大學材料科學與工程學院,北京100124)
合成了兩個新的銀(Ⅱ)配合物,[Ag2Br2(DPEphos)2(dppe)](1)和[Ag(DPEphos)(dppe)]NO3(2)(DPEphos=雙[(2-二苯膦基)苯基]醚; dppe=1,2-雙(二苯膦)乙烷),通過紅外光譜、X-射線單晶衍射、核磁共振氫譜、磷譜和熒光光譜進行分析和表征。1是由AgBr, DPEphos和dppe以2∶2∶1的比例混合反應得到的雙核化合物,dppe通過2個P原子橋連2個Ag原子,而2是由AgNO3, DPEphos和dppe以1∶1∶1的比例混合反應得到的簡單的單核化合物,Ag原子與DPEphos和dppe配體螯合。在配合物2的膦譜中,存在4個分裂峰(雙峰或者三重峰)。熒光光譜表明所有的發(fā)射峰均源于配體中的π-π*躍遷。
雙[(2-二苯膦基)苯基]醚;1,2-雙(二苯膦)乙烷;銀;熒光
0 Introduction
More and more interests are focused on the group 11 metal complexes owing to their rapid developments in their structural diversity[1-2]and intriguing optical properties[3-4].In recent years,many efforts have beendone to get a deeper research on their potential applications of the closed-shell d10metals complexes, such as luminescent-based chemical sensors[5],organic light emitting diodes[6],catalysis[7-8]and sensitizers in solar-energy conversion[9].The Ag(Ⅱ)atom commonly adopts four-coordinated mode indicating a distorted tetrahedron.
The Ag(Ⅱ)compounds containing P-donor ligands attractmuchattentionduetotheirlowcost, nontoxicity and luminescence.Among a number of P-donor ligands,the type Ph2P(CH2)nPPh2ligand is used toformextensiveandvariedcoordination compounds[10].The coordination behavior(chelating or bridging mode)primarily depends on the length of the methylene chain between the two P donors[11-12].In addition,the transition metal chemistry and catalytic utility of the bis[2-(diphenylphosphino)phenyl]-ether (DPEphos)have been extensively studied by van Leeuwen and co-workers[13]and others[14-15].Recently, considerable attention has been paid on the coordination architectures of metal-DPEphos complexes,such as ruthenium(Ⅱ)[16],gold(Ⅱ)[17]and silver(Ⅱ)complexes[18].
Last year,our group reported a series of Ag(Ⅱ)complexes based on mixed phosphine ligands and splitting phenomenon of31P NMR signals[19].After that, we synthesized two novel silver(Ⅱ)complexes(Scheme 1),Ag2Br2(DPEphos)2(dppe)](1)and[Ag(DPEphos) (dppe)]NO3(2),(DPEphos=bis[2-(diphenylphosphino) phenyl]ether,dppe=bis(diphenylphosphino)ethane). They have been characterized by IR,single-crystal X-raydiffraction,1H/31PNMRspectroscopyand fluorescence spectrum.We also discussed the role of the impact of anion and the proportion of different diphosphine ligands in the synthesis.Furthermore,a similar splitting phenomenon in31P NMR was found.
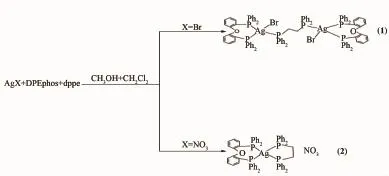
Scheme 1Routine of synthesis for complexes 1 and 2
1 Experimental
1.1 Materials and measurements
All chemical reagents are commercially available and used without furthermore treatment.FTIR spectra (KBr pellets)were measured on a Perkin-Elmer Infrared spectrometer.C,H and N elemental analysis were carried out on an Elementar Vario MICRO CUBE (Germany)elementalanalyzer.Room-temperature fluorescence spectra were measured on F-4500 FL Spectrophotometer.1H NMR and31P NMR was recorded at room temperature with a Varian VNMRS 600MHz spectrometer and31P NMR was recorded at room temperaturewithaVarianVNMRS243MHz spectrometer.
1.2 Synthesis of[Ag2Br2(DPEphos)2(dppe)](1)
Complex 1 was prepared by the reaction of AgBr (0.037 7 g,0.2 mmol)with DPEphos(0.107 3 g,0.2 mmol)and dppe(0.039 9 g,0.1 mmol)in the mixed solvents of 5 mL CH2Cl2and 5 mL CH3OH.The mixture was stirred for 6 hours and filtered.Colorless crystals were obtained from the filtrate after standing at room temperature for several days.Yield:49%.Element analysis Calcd.(%)for C98H80Ag2Br2O2P6:C,63.53;H, 4.32;Found(%):C,62.13;H,4.60.IR data(cm-1,KBr pellets):3 427m,3 048w,1 621w,1 562w,1 480w, 1 458w,1 433s,1 257w,1 218m,1 094w,1 025w, 741s,693s,510m,497m,469w.1H NMR(600 MHz, CDCl3,298 K):δ 7.3~7.1(m,CHbenzene),1.6(s,CH2).31P NMR(243MHz,CDCl3,298 K):δ-4.0(br),-11.0(br,d).
1.3Synthesis of[Ag(DPEphos)(dppe)]NO3(2)
The preparation of complex 2 was similar to 1 except that the salt was changed to AgNO3(0.034 1 g,0.2 mmol).Colorless crystals were obtained with a yield of 57%.Element analysis Calcd.(%)for C62H52AgNO4P4:C, 67.22;H,4.70;N,1.26;Found(%):C,66.67;H,4.72;N, 1.31.IR data(cm-1,KBr pellets):3 435w,3 052w, 1633w,1586w,1564w,1480w,1460w,1434s,1384s, 1 341m,1 258w,1 215m,1 095w,744s,495s,512m, 477w.1H NMR(600 MHz,CDCl3,298 K):δ 7.4~6.8(m, CHbenzene),1.7(s,CH2).31P NMR(243 MHz,CDCl3,298K): δ3.2(dd),-2.7(dt),-7.2(dt),-8.8(dd)
1.4 Structure determination
Single crystals of the title complexes were mounted on a Bruker Smart 1000 CCD diffractometer equipped with a graphite-monochromated Mo Kα(λ=0.071 073 nm)radiation at 298 K.Semi-empirical absorption corrections were applied using SABABS program.All the structures were solved by direct methods using SHELXS program of the SHELXTL-97 package and refined with SHELXL-97[19].Metal atom centers were located from the E-maps and other non-hydrogen atoms were located in successive difference Fourier syntheses. The final refinements were performed by full matrix least-squaresmethodswithanisotropicthermal parameters for non-hydrogen atoms on F2.The hydrogen atoms were generated geometrically and refined with displacement parameters riding on the concerned atoms.
Crystallographic data and experimental details for structural analysis are summarized in Table 1,and selected bond lengths and angles of complexes 1~2 are summarized in Table 2.
CCDC:1043375,1;1043376,2.
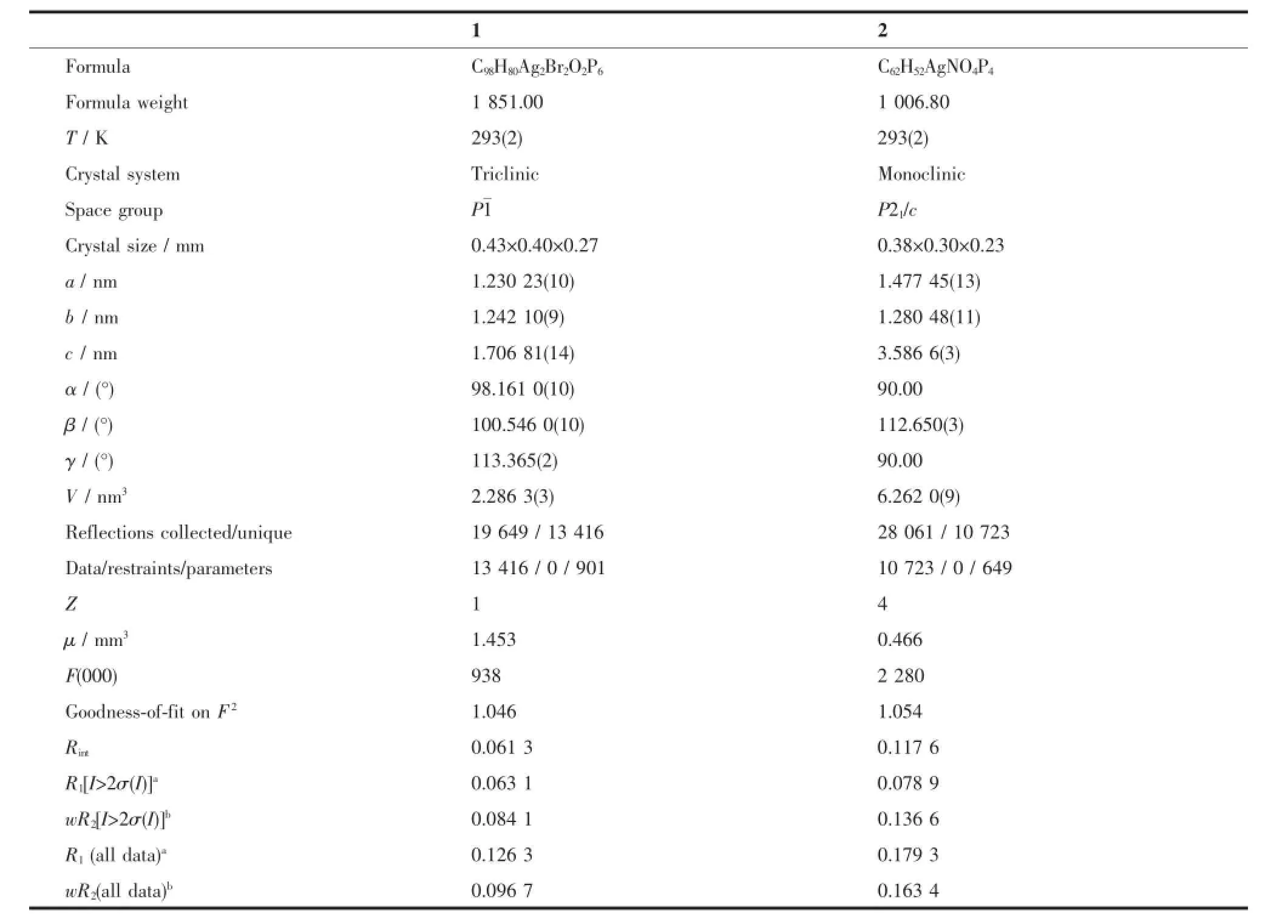
Table 1Crystallographic data for complexes 1~2
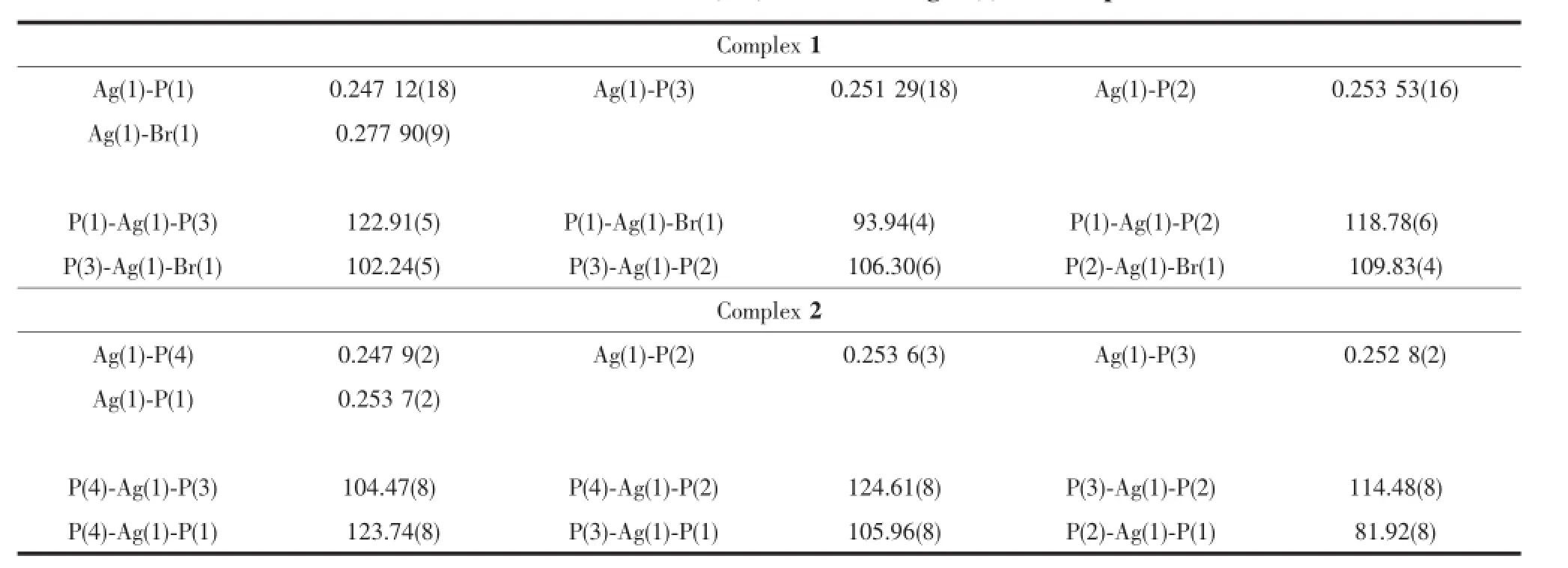
Table 2Selected bond distances(nm)and bond angles(°)for complexes 1~2
2 Results and discussion
2.1 Syntheses of the single crystals
As we all know,different solvents can also affect the structures of the products.Last year,our group synthesized two complexes[Ag2Cl2(DPEphos)2(dppe)]· 2CH3OH·DMFand[Ag2Br2(DPEphos)2]·2DMFin differnt solvents[20].Itshould be mentioned that different complexes are synthesized in the same molar ratio and solvent,when the anion is different halogen atom.It isn′t found that Ag(Ⅱ)atom is connected to dppe ligand in any mode.Now,the complex 1 is prepared in the mixed solution environment(5 mL CH3OH and 5 mL CH2Cl2).The crystal structure is similar with[Ag2Cl2(DPEphos)2(dppe)]·2CH3OH·DMF. Itisprovedthatsolventsareimportanttothe formation of the coordination compounds.
Inaddition,threesimplemono-nuclear complexes are prepared in the same proportion and solvent.The complex 2 is prepared by AgNO3,dppe, and DPEphos in 1∶1∶1 molar ratio generating a mononuclear complex.While,thecomplex[Ag2(NO3)2(DPEphos)2(dppe)]is synthesized by AgNO3,dppe,and DPEphos in 2∶1∶2 molar ratio generating a binuclear complex[20].By comparing to complex 2,we find dppe ligandcanactasbridgingorchelatingligand depending on the proportion.
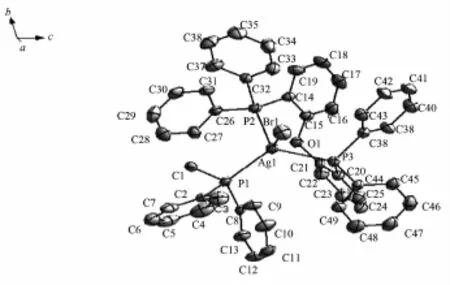
Fig.1Asymmetric unit of complex 1
2.2 Description of the Crystal Structure
Complex 1 is crystallized in the triclinic crystal systemand contains acrystallographiccenter of symmetry(Fig.1).Each asymmetric unit is composed of half of a dppe ligand,a bromine atom and a DPEphos ligand chelating a Ag(Ⅱ)atom.In complex 1, two silver atoms are linked by bridging dppe ligand toform a binuclear structure.The Ag(Ⅱ)metal adopts four-coordinatedmodeestablishingadistorted tetrahedral geometry.Thegeometryaroundmetal center is distorted tetrahedral because of the angles in the range of 93.94(4)°~122.91(5)°.The Ag-P(dppe) bond distance is 0.247 12(18)nm in accord with that of[Ag2Cl2(DPEphos)2(dppe)]·2CH3OH·DMF[20],indicating that free solvent molecular and halogen atom are influcented.The Ag-P(DPEphos)bond distances (0.251 29(18)nm and 0.253 53(16)nm)are simliar comparedwiththoseofcomplexesinprevious literature[19].The bite angles of the DPEphos ligand is 106.30(6)°,which is 11.10°and 11.80°less than the same angle in the complex[Ag2(κ2-P,P-DPEphos)2(μ-OTf)2][21]and Ag2I2(DPEphos)2[22].
Complex 2 is simple mono-nuclear complex formed withdistinctly soft Ag(Ⅱ),dppe ligand,DPEphos ligand and anion(Fig.2).Each Ag(Ⅱ)atom is fourcoordinated,attached to two P atoms from a chelating dppe ligand and two P atoms from a chelating DPEphos ligand.In Complex 2,the angles around Ag(Ⅱ)atom ranging from81.92(8)°to 124.61(8)° indicatethatthegeometryaroundAgatomis distortedlytetrahedral.Thebiteanglesofthe DPEphos ligand is 104.47(8)°,which is smaller than the same angle in the complexes[Ag(POP)2]+[23]and [Ag(DPEphos)(PY2SH)2]NO3[24].The Ag-P(DPEphos or dppe)bond distance is similar with that in[Ag (DPEphos)(dppe)]BF4[19].
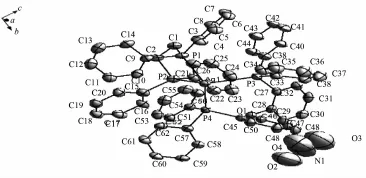
Fig.2Molecular structure of complex 2
2.3 Infrared spectroscopy
The infrared spectra of complexes 1~2 show the absorptions around 1 434 cm-1are due to C-C stretch vibrationofthephenylringsandthemiddle absorptions around 3 052 cm-1are caused by C-H vibration of the phenyl rings.The absorptions of the C-O-C stretch vibration are around 1 215 cm-1.The absorptions in 1 384 cm-1and 1 341 cm-1are derived from nitrate(NO3-)in complex 2.
2.431P NMR spectroscopy
The31P NMR spectra of complexes 1~2 and two kinds of phosphine(DPEphos and dppe)ligands have been measured at room temperature in CDCl3.In31P NMR spectra of two kinds of phosphine ligands,the31P NMR spectra show a singlet resonance peak.The P signals of free DPEphos and free dppe exist at -16.9 and-12.6,respectively.
Interestingly,the31P NMR spectrum of 2 consists of two pairs of well resolved doublets centered at 3.2 and-8.8.Furthermore,31P NMR spectrum of 2 consists of two pairs of triplets centered at-2.7 and-7.2.Every peak of the triplets changes into doublet((Fig.3).
2.5 Fluorescence Spectrum
The luminescent emission spectra of complexes 1~2 in the solid state at room temperature are obtained(Fig.3).In the fluorescence emission spectra of solid dppe and DPEphos,the emission peaks are found at 435 nm(λex=357 nm for dppe)and 453 nm (λex=317 nm for DPEphos),respectively[19].In the fluorescence emission spectra of 1~2,the emission peaks are found at 447 nm(λex=372 nm for 1)and 433nm(λex=362 nm for 2).The emissions of complex 1 exhibits a red-shift distinctly,compared to that of the corresponding free ligand dppe.The emissionpeak is similar to that of dppe,which indicates that the origin of the emission involves emissive state derived from ligand-centered π-π*transition.
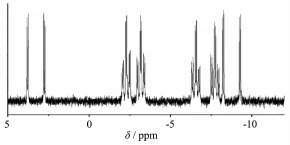
Fig.331P NMR spectrum of 2 in CDCl3
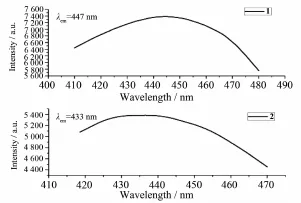
Fig.4Solid-state emission spectra of 1~2 at 298 K
3 Conclusions
Two new Ag(Ⅱ)complexes of phosphine-containing ligands(DPEphos and dppe)have been synthesized and characterizedbyelementalanalysis,IR,X-ray diffraction,fluorescence,1HNMR,31PNMR spectroscopy.Structure analyses show that two silver atoms are linked by bridging dppe ligand and chelated two DPEphos ligands to form a binuclear structure in complex 1.While,complex 2 is a simple mononuclear structure.Each silver atom is chelated a dppe ligand and a DPEphos ligand.In the31P NMR spectra of 2, there are four splitting signals(doublets or triplets).The luminescent spectra show that the origin of these emissions all involves emissive state derived from ligand centered π-π*transition.We hope our results could offer new strategy for the characterization and design of metalorganic compounds.
[1]Kim E,Lee H,Noh T H,et al.Cryst.Growth Des.,2014,14: 1888-1894
[2]Yue C Y,Yan C F,Feng R,et al.Inorg.Chem.,2009,48: 2873-2879
[3]Rajput G,Yadav M K,Drew M G,et al.Inorg.Chem.,2015, 54:2572-2579
[4]Nitsch J,Kleeberg C H,Fr?hlich R,et al.Dalton Trans., 2015,44:6944-6960
[5]Demas J N,Graff B A.Coord.Chem.Rev.,2001,211:317 -351
[6]Minaev B,Jansson E,?gren H.J.Chem.Phys.,2006,125: 234704/1-18
[7]Tsubomura T,Sakai K.Coord.Chem.Rev.,1998,171:107-113
[8]Wang X X,Liu Y G,Hecke K V,et al.Z.Anorg.Allg. Chem.,2015,641:903-910
[9]Felder D,Nierengarten J F,Barigelletti F,et al.J.Am.Chem. Soc.,2001,123:6291-6299
[10]Effendy,Nicola C,Pettinari C,et al.Inorg.Chim.Acta, 2006,359:64-80
[11]Qiu Q M,Huang X,Zhao Y H,et al.Polyhedron,2014,83: 16-23
[12]Cui L N,Li Z F,Jin Q H,et al.Inorg.Chem.Commun., 2012,20:126-130
[13]Kranenburg M,Burgt Y,Kamer P,et al.Organometallics, 1995,14:3081-3089
[14]Kuang S,Cuttell D G,McMillin D R,et al.Inorg.Chem., 2002,41:3313-3322
[15]Kuang S M,Fanwick P E,Walton R A.Inorg.Chem., 2002,41:405-411
[16]Venk ateswaran R,Mague J T,Balakrishna M S.Inorg. Chem.,2007,46:809-817
[17]Partyka D V,UpdegraffⅢJ B,Zeller M,et al.Dalton Trans.,2010,39:5388-5397
[18]Balakrishna M S,Venkateswaran R,Mobin S M.Inorg. Chim.Acta,2009,362:271-276
[19]Sheldrick G M.SHELXS-97 and SHELXL-97,University of G?ttingen,G?ttingen,Germany,1997.
[20]Gao S,Li Z F,Liu M,et al.Polyhedron,2014,83:10-15
[21]BalakrishnaMS,VenkateswaranR,MobinSM. Polyhedron,2008,27:899-904
[22]Freudenmann D,Feldmann C.Inorg.Chim.Acta,2011, 375:311-313
[23]Adrien K,Omar M,Gianluca A,et al.Eur.J.Inorg.Chem., 2014,2014:1345-1355
[24]George C,Philip J C,Paraskevas A.Polyhedron,2012,31: 502-505
Syntheses and Characterization of Two Luminescent Silver(Ⅱ)Complexes Based on Mixed Phosphine Ligands
ZHANG Yan-Ru1WANG Meng-Qin1CUI Yang-Zhe1LIU Min2LI Zhong-Feng1JIN Qiong-Hua*,1
(1Department of Chemistry,Capital Normal University,Beijing 100048,China)
(2College of Materials Science and Engineering,Beijing University of Technology,Beijing 100124,China)
Two novel silver(Ⅱ)complexes[Ag2Br2(DPEphos)2(dppe)](1)and[Ag(DPEphos)(dppe)]NO3(2)(DPEphos= bis[2-(diphenylphosphino)phenyl]ether,dppe=bis(diphenylphosphino)ethane)have been synthesized and characterized by IR,single-crystal X-ray diffraction,1H NMR,31P NMR spectroscopy and fluorescence spectra.1 is comprised of AgBr,DPEphos and dppe in 2∶2∶1 molar ratio generating a binuclear complex.The dppe ligand bridges two Ag atoms through two P atoms in 1.While,2 is obtained by the reactions of AgNO3,DPEphos and dppe in 1∶1∶1 molar ratio generating a sample mono-nuclear complex.The Ag(Ⅱ)atom is chelated by DPEphos and dppe ligand.In the31P NMR spectra of 2,there are four splitting signals(doublets or triplets).The luminescent spectra show that the origin of these emissions all involves emissive state derived from ligand centered π-π*transition.CCDC:1043375,1;1043376,2.
bis[2-(diphenylphosphino)phenyl]ether;bis(diphenylphosphino)ethane;silver(Ⅱ);fluorescence
O614.122
A
1001-4861(2015)10-2089-06
10.11862/CJIC.2015.274
2015-06-28。收修改稿日期:2015-07-12。
國家自然科學基金(No.21171119),863國家高技術研究發(fā)展計劃(No.2012AA063201),北京教育委員會基金(No.KM201210028020),北京市優(yōu)秀人才項目(No.2010D005016000002),北京市自然科學基金(No.7122015)資助項目。
*通訊聯(lián)系人。E-mail:jinqh@cnu.edu.cn;會員登記號:S06N3669M1105。

