Preparation of Au-OVs-BiOBr-P25 Z-scheme photocatalyst and its photocatalytic performance in overall water splitting
FANG Wei-li,WANG Liang,LI Chun-hu
(College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China)
Abstract:Z-scheme photocatalyst holds great promise in photocatalytic H2 evolution. In this work, a ternary Au-OVs-BiOBr-P25 Z-scheme photocatalyst with oxygen vacancies was successfully prepared, in which Au nanoparticles were used as the electron mediators to introduce into BiOBr and P25. The photocatalytic activity of this ternary photocatalyst was evaluated by overall water splitting. The H2 evolution rate of Au-OVs-BiOBr-P25 achieves an amazing value of 384 μmol/(g·h) under UV-vis irradiation. UV-vis DRS and transient photocurrent spectra revealed that the enhanced photocatalytic activity of Au-OVs-BiOBr-P25 was mainly attributed to its widened photo-response range and effective carrier separation. Furthermore, the photocatalytic mechanism was systematically studied by EPR and Photoelectrochemical measurements, which indicated that the overall water splitting occurred through the two-electron pathway. This result will provide us new ideas for developing more efficient photocatalysts for photocatalytic H2 evolution.
Key words:Au-OVs-BiOBr-P25;overall water splitting;oxygen vacancy;Au nanoparticles;H2O2
Photocatalytic H2evolution has become an effective way to solve energy and environmental issues. Since Honda and Fujishima[1]discovered the capability of TiO2electrodes in H2evolution via decomposing water in 1972, photocatalytic H2evolution has attracted a great deal of attentions.However, photocatalytic semi-decomposition of water requires the addition of sacrificial agents (e.g.methanol, sodium sulfite, etc.), and the conventional overall water splitting (2H2O→O2+2H2) is dynamically slow because it occurs via the four-electron pathway.To solve these problems, Liu et al.[2]designed and prepared a metal-free CDots-C3N4nanocomposite to accomplish the overall water splitting via the kinetically dominated two-electron pathway(2H2O→H2O2+H2). Since then, the overall water splitting via this pathway has attracted widespread attention[3]. For instance, Cao et al.[4,5]reported Pt/TiO2photocatalysts for photocatalytic evolution of H2O2and H2via a two-electron pathway.
Z-scheme photocatalysts have great advantages in improving the light utilization efficiency and the separation efficiency of photogenerated carriers[6],promoting high photocatalytic oxidation and reduction performance with combined two suitable photocatalysts[7,8]. However, there are few studies on overall water splitting via the two-electron pathway,TiO2(P25) is the most extensively studied conventional photocatalysts[9], BiOBr displays good visible-light responsive activity and powerful oxidization capacity[10,11]. As BiOBr/TiO2heterojunction can effectively broaden the visible light response range and significantly improve the photocatalytic activity, it has been widely reported[12,13]. For instance, Rashid et al.[14]synthesized butterfly clusters of laminar BiOBr on the surface of TiO2nanoparticles in situ for the photocatalytic degradation of ciprofloxacin and the results showed the ciprofloxacin degradation by BiOBr/TiO2was 5.2 and 9.4 times of TiO2and BiOBr.
Oxygen vacancies(OVs) can significantly improve the photocatalytic activity of photocatalysts[15,16]. The introduction of OVs can adjust the structure of the energy band to shift the Fermi energy level upward,increase the range of light absorption, and promote carrier separation by trapping electrons or holes[17].Meanwhile, OVs can also provide more activation sites for the reaction. Li et al.[18]designed BiOBr with OVs on exposed (001) surfaces, which can achieve effective nitrogen fixation in visible light. Moreover, surface plasmon resonance (SPR)-induced chemical reaction can effectively contribute to the conversion of solar energy to chemical energy[19]. As a hydrogen-out photocatalyst, Au nanoparticles(AuNPs) is more stable than the transition metal, Recently, Ma et al.[20]synthesized the Au-Cu2O heterojunction and greatly improved the photocatalytic overall water splitting.Meanwhile, the synergistic effect of OVs and AuNPs can further improve the photocatalytic activity[21].
Herein, we have successfully constructed a novel Au-OVs-BiOBr-P25 Z-scheme photocatalyst by hydrothermal synthesis and solution reduction method.The as-prepared photocatalyst greatly improves the photocatalytic overall water splitting performance via a two-electron reaction pathway. The mechanism of overall water splitting over Au-OVs-BiOBr-P25 was systemically explored by various characterization techniques. This new ternary photocatalyst has promising applications for photocatalytic H2evolution.
1 Experimental
1.1 Materials
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O,AR), Potassium Bromide (KBr, AR), Titanium dioxide(P25), Sodium borohydride (NaBH4, AR), Citric acid(C6H8O7, AR), Chloroauric acid solution (HAuCl4,1%),NaOH solution (0.2 mol/L). All chemical agents used in this study were of analytical grade as received without further purification.
1.2 Synthesis of BiOBr-P25
In a typical experiment, 5 mmol of Bi(NO3)3·5H2O was dissolved in 40 mL of deionized water and then 5 mmol of KBr and 5 mmol of P25 were added to above solution with continuous stirring for 5 h. The mixture was immediately transferred into a 50 mL Teflon-lined stainless-steel autoclave, which was heated up and maintained at 160 °C for 16 h. After being cooled down to room temperature, the autoclave was washed with deionized water for several times to collect all the products. The reaction products were extracted by filtration, and was dried at 60 °C for 12 h.For comparison, the pure BiOBr were prepared as the above same way without the addition of P25.
1.3 Synthesis of OVs-BiOBr-P25
1 g of BiOBr-P25 was dispersed in 40 mL of 30 mmol/L NaBH4solution. Then, the suspension was stirred for 30 min. The precipitate was collected and washed several times with ethanol and deionized water,respectively. Ultimately, OVs-BiOBr-P25 photocatalyst was obtained by grinding after drying in an oven at 60°C for 12 h.
1.4 Synthesis of Au-OVs-BiOBr-P25
Au-OVs-BiOBr-P25 was synthesized by anin-situreduction method. 0.5 g OVs-BiOBr-P25 were mixed in deionized water, and then 0.44 mL HAuCl4solution(1%) was added. The mixture was stirred at room temperature for 12 h. Citric acid solution was added into the mixture at 25 °C and hold for 30 min. The precipitate was separated by filtration and washed with deionized water for several times. Finally, the Au(NPs)-loaded catalyst was dried in a vacuum oven at 60 °C for 12 h. The synthesis scheme of the photocatalyst is shown in Figure 1.
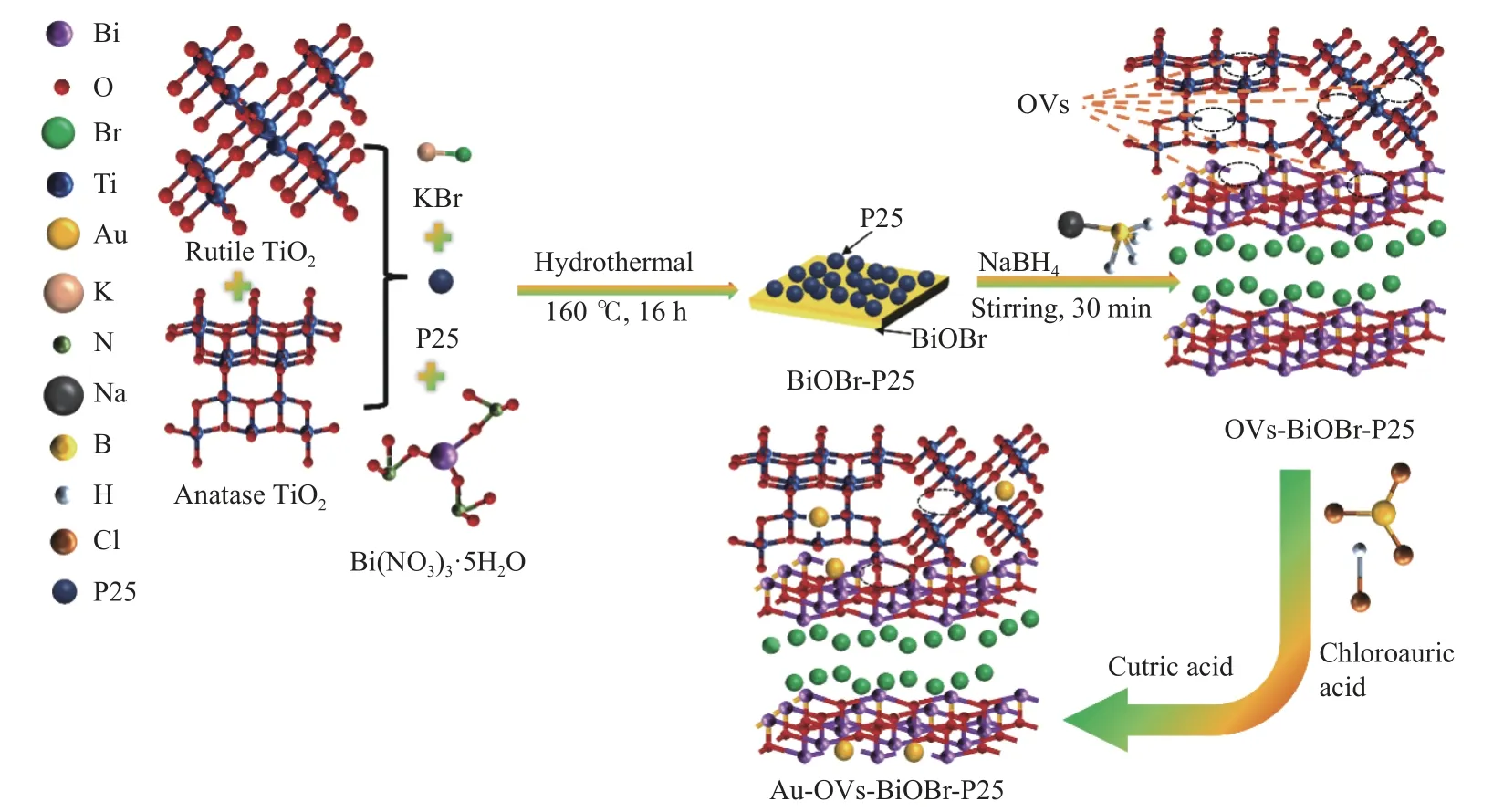
Figure 1 Synthesis process of Au-OVs-BiOBr-P25
1.5 Characterizations
The crystal structure of photocatalyst was obtained by X-ray diffraction analysis (XRD, Bruker D8 Advance with CuKα (λ=0.154 nm)). The elementary composition was investigated by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250X, America). The microscopic morphology,structure and size of photocatalyst were analyzed by scanning electron microscope (SEM, Hitachi S-4800,Japan) and transmission electron microscopy (TEM,JEM-2100, Japan). UV-vis diffusion reflectance spectrums (UV-vis DRS) of photocatalyst were obtained via a UV-vis spectrophotometer (UV-3600,Shimadzu, Japan). The OVs in the photocatalyst was performed with an electron spin resonance spectrometer (EPR, A300-10/12, Bruker, Germany).The ESR spectra were taken on a Bruker ESP-300E spectrometer and X-band (9.8 GHz).
1.6 Overall water splitting test
The photocatalytic H2evolution was carried out in Labsolar-6A online photocatalytic analysis system(Beijing Perfect Light Technology Co. LTD.). In a typical experiment, 50 mg photocatalyst was dispersed in 100 mL deionized water. A vacuum was applied to the system for 15 min before the start of the experiment to ensure that the system was free of impurity gases.The reaction system was irradiated with a 300W Xelamp. A gas chromatograph (GC-7806) was used to separate and analyze the hydrogen produced by the photocatalytic reaction. H2O2was determined by a hydrogen peroxide detector (Q45H/84 H2O2analyzer,America Qcontums). In this experiment, the cooling water circulation system was used to keep the temperature of the system at room temperature (25 °C).
1.7 Photoelectrochemical measurements
The photocurrent intensity and Mott-Schottky plot were determined by CHI660e electrochemical workstation. For each typical photoelectrochemical test, as-prepared samples deposited on FTO, Pt wire electrode and Ag/AgCl (saturated KCl) were used as work electrode, counter electrode and the reference electrode, NaSO4(0.1 mol/L) solution as electrolyte,respectively.
2 Result and discussion
2.1 Crystalline phase and Microstructures
The structures of all the photocatalysts were investigated by XRD. As can be seen from Figure 2, a mixed phase of rutile and anatase can be observed for P25. Anatase TiO2(JCPDS No. 21-1272) is the dominant phase, with diffraction peaks at 25.3°, 37.8°,48.0°, 54.1°, 55.1° and 62.7°. In addition, P25 had a weak diffraction peak at 27.5°, which is corresponding to the (110) crystal plane of the rutile crystal phase(JCPDS No.21-1276)[22]. The diffraction peaks of BiOBr are well matched to the tetragonal phase of BiOBr (JCPDS No.09-0393)[23]. For the two samples of OVs-BiOBr-P25 and Au-OVs-BiOBr-P25, some similar diffraction peaks are shown, but these peaks intensity become weaker compared with BiOBr and P25, which indicates that the decrease of the crystallinity derived from OVs[24]. Moreover, the peak of Au NPs cannot be found in the diffraction pattern of Au-OVs-BiOBr-P25, attributed to lower Au loading and higher dispersion[25]. In addition, the peaks with 2θvalues at 27.2° could be assigned to the (012)crystalline surface of Bi (JCPDS No.44-1246)[26], which suggested that Bi had been reduced from BiOBr with the presence of NaBH4as reducing agent.
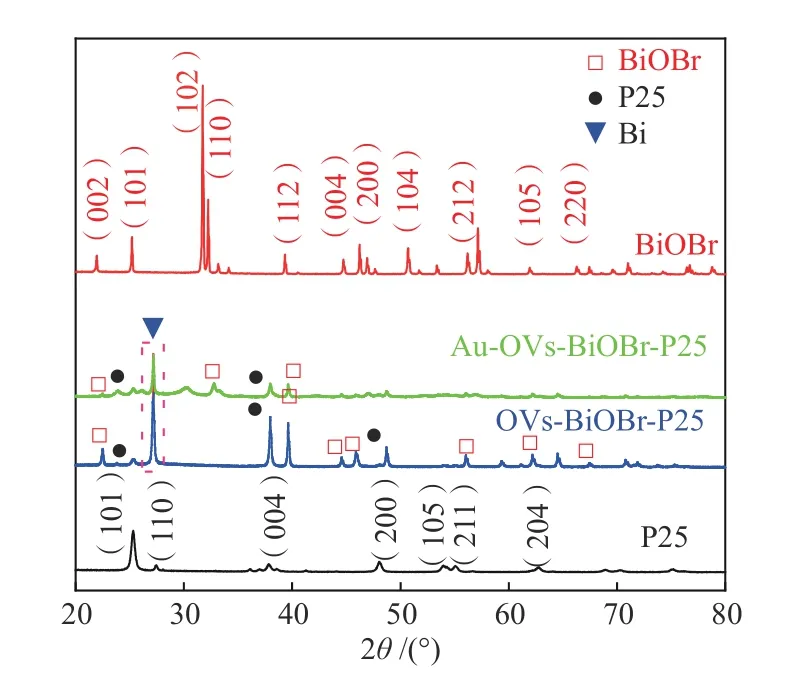
Figure 2 XRD patterns of BiOBr, P25, OVs-BiOBr-P25 and Au-OVs-BiOBr-P25
XPS was carried out to further determine the surface chemical compositions of Au-OVs-BiOBr-P25.As shown in Figure 3, it can be obviously verified that Au-OVs-BiOBr-TiO2consists of Bi, O, Br, Ti and Au elements. The signal peak observed at 284.6 eV belongs to C 1s. Figure 3(b) clearly shows the characteristic peaks at the binding energy of 457.8 and 464.6 eV corresponding to the Ti?O bonding for Ti 2p3/2and Ti 2p1/2, respectively. We can find that the binding energy has shifted from 458.3 to 457.8 eV,indicating that more Ti4+transitions to the Ti3+and also demonstrating the formation of OVs[27]. The peak at 467.6 eV corresponds to Bi 4d. The XPS high resolution spectra of O 1sis shown in Figure 3(c). The characteristic peaks at 529.1 and 530.3 eV correspond to Bi?O and Ti?O?Ti bonds, respectively. The XPS spectra at Bi 4f(Figure 3(d)) presents two characteristic peaks at 158.4 and 163.7 eV corresponded to the Bi3+of Bi 4f7/2and Bi 4f5/2[28,29], respectively. In addition, two signal peaks at 156.4 and 161.4 eV were found in Figure 3(d), which matched Bi0in Au-OVs-BiOBr-P25, further indicated that Bi3+was reduced to Bi[30]. In the XPS spectrum of Au (Figure 3(e)), there are two characteristic peaks at 83.1 and 86.8 eV attributed to Au 4f7/2and 4f5/2[31]. In the EPR spectrum (Figure 3(f)),it can be find that both Au-OVs-BiOBr-P25 and OVs-BiOBr-P25 exhibit much stronger EPR signal atg=2.003 compared to BiOBr and P25, attributed to the formation of a larger number of OVs for the two samples.
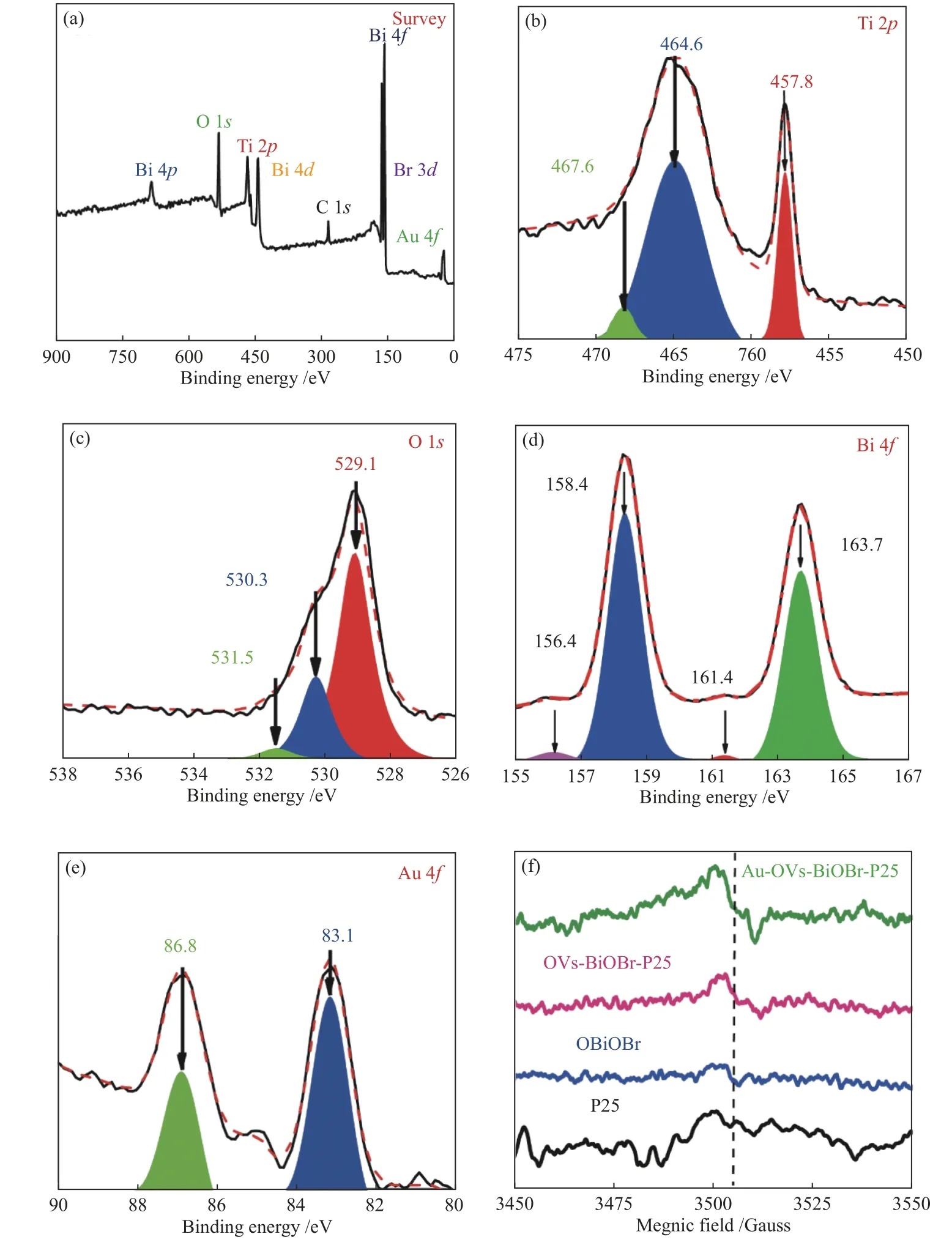
Figure 3 XPS spectra of Au-OVs-BiOBr- P25 (a) survey spectra, high resolution spectra of (b) Ti 2p spectrum, (c) O 1s spectrum,(d) Bi 4f spectrum, (e) Au 4f spectrum, (f) EPR spectra of BiOBr, P25, Au-OVs-BiOBr and Au-OVs-BiOBr-P25
The morphology and structures of BiOBr, P25,OVs-BiOBr-P25 and Au-OVs-BiOBr-P25 were characterized by SEM. In Figure 4(a), P25 with a diameter of about 50 nm can be observed and BiOBr presents a unique layered structure with each layer of about 2.0 μm broad (Figure 4(b)). After being modified with P25, the diameter of BiOBr decreased obviously(Figure 4(c) and Figure 4(d)), because some P25 Nps were deposited on the surface of BiOBr. The microstructural of Au-OVs-BiOBr-P25 was further analyzed by TEM and HRTEM. From the image(Figure 4(e)), we can clearly find that the heterojunction structure between BiOBr and P25 was successfully prepared and formed. In addition, The HRTEM image shows a lattice spacing of 0.228 nm assigned to the (112) face of BiOBr, the (101) face(0.352 nm) of anatase TiO2and the (110) face (0.325 nm) of rutile TiO2[32], respectively. As shown in Figure 4(f), it can be derived that the particle size of Au NPs is about 2 nm, which are distributed on the surface of Au-OVs-BiOBr-P25. Furthermore, the energy dispersion X-ray (EDX) element mapping of O, Ti, Br, Au and Bi further confirmed the formation of heterogeneous structures. EDX mapping of Au-OVs-BiOBr-P25(Figure 5) shows that the hetero-structure is composed of Au, Ti, Bi, Br and O elements.
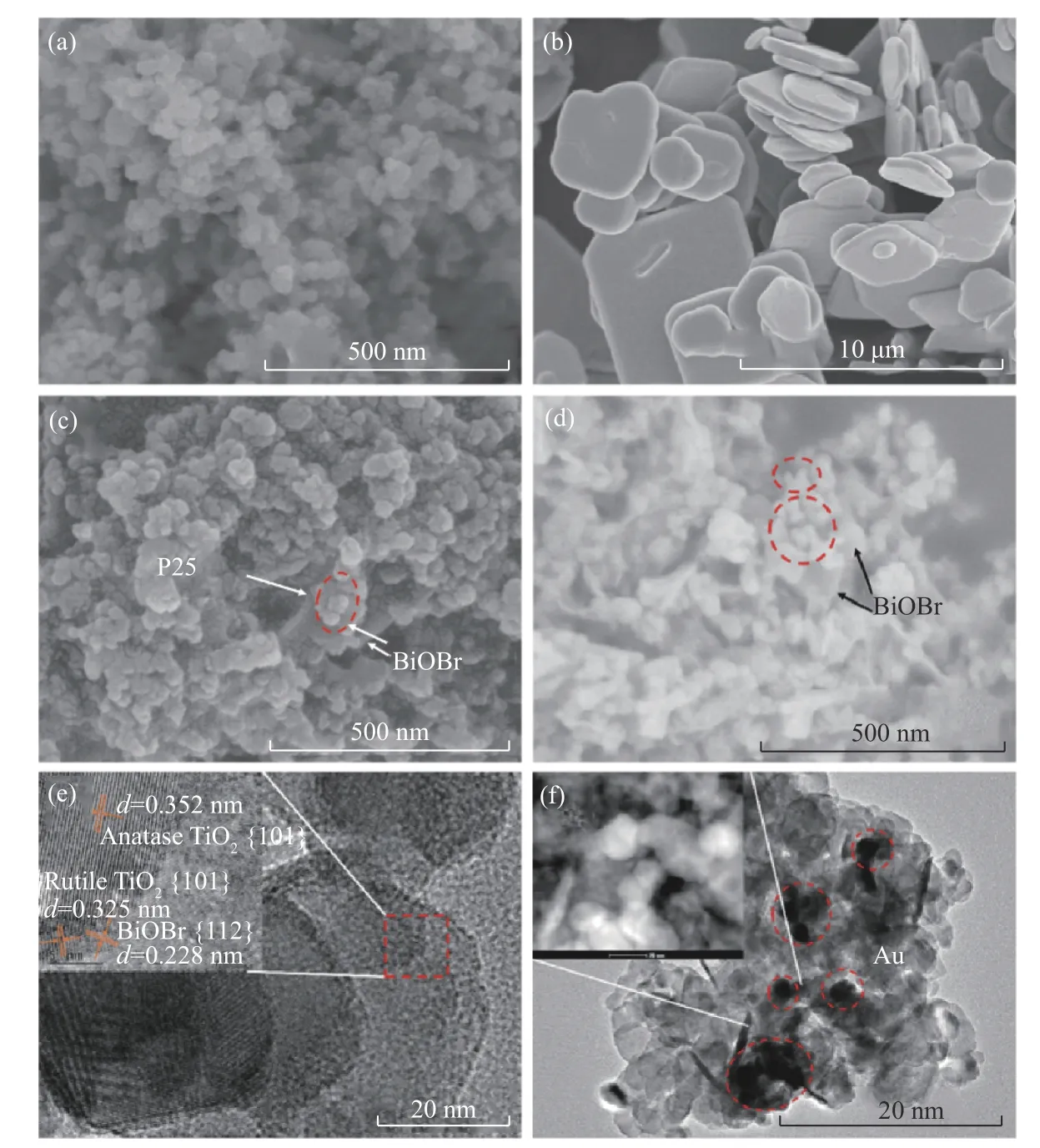
Figure 4 SEM images of (a) P25, (b) BiOBr, (c-d) OVs-BiOBr- P25, (e) HRTEM image,(f) TEM image (inset is STEM) of Au-OVs-BiOBr-P25
2.2 Optical and electrical properties
The optical properties of BiOBr, P25, OVs-BiOBr-P25 and Au-OVs-BiOBr-P25 were characterized by UV-vis DRS. In Figure 6, P25 showed optical absorption below 380 nm, belonged to the ultraviolet absorption region, while the absorption of BiOBr implied visible light absorption until 440 nm.With the introduction of oxygen vacancy into the photocatalyst, OVs-BiOBr-P25 exhibited a higher light absorption ability in visible-light region. This main reason is that the color of OVs-BiOBr-P25 turns black after introducing oxygen vacancy, which enables OVs-BiOBr-P25 to have higher light absorption performance in the visible region. The absorption edge of Au-OVs-BiOBr-P25 exhibited strong red shift compared with P25 and BiOBr.
As we all known, the charge separation and transfer efficiency have great influences on the photocatalytic activity. To investigate the separation of photogenerated carriers, the transient photocurrent responses of all the photocatalysts were measured and shown in Figure 7. In Figure 7, the photocurrent intensities for both Au-OVs-BiOBr-P25 and OVs-BiOBr-P25 were significantly higher than those of P25 and BiOBr, Au-OVs-BiOBr-P25 has the highest photocurrent intensity. The above phenomenon illustrates that OVs and Au NPs can inhibit the photogenerated electron-hole pairs recombination, thus improving the photocurrent intensity.
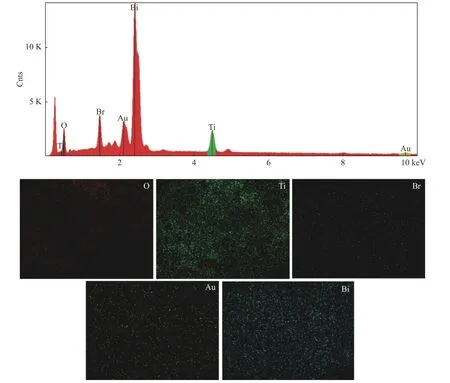
Figure 5 Element mapping of Au-OVs-BiOBr-P25
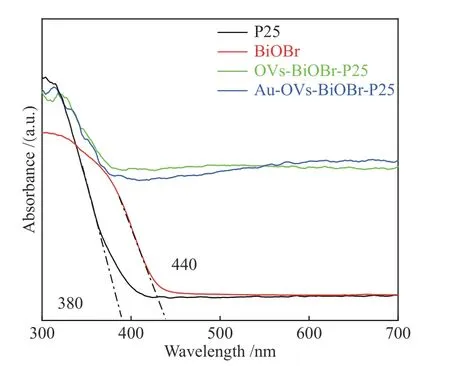
Figure 6 UV-vis diffuse reflection spectra of BiOBr, P25,OVs-BiOBr-P25 and Au-OVs-BiOBr-P25
2.3 Photocatalytic activity test
The photocatalytic activities of P25, BiOBr, OVs-BiOBr-P25 and Au-OVs-BiOBr-P25 were examined by overall water splitting. Figure 8(a) shows that the pure BiOBr showed no hydrogen production during photocatalytic reaction process, and 97 μmol/(g·h) H2was generated over P25. Meanwhile, the hydrogen production over OVs-BiOBr-P25 reached 301 μmol/(g·h), which is lightly lower than that of Au-OVs-BiOBr-P25 (384 μmol/(g·h)), indicating that both the presence of OVs and the loading of Au NPs improved the photocatalytic performance. As shown in Figure 7(b),H2O2was detected in the process of the photocatalytic reaction over Au-OVs-BiOBr-P25, and no H2O2was formed in the reaction solution with OVs-BiOBr-P25 as a photocatalyst. It can be seen from the Figure 8(b)that the yield ratio of H2and H2O2deviates from 1.This may be due to the SPR effect of Au, Au only provides more thermal electrons to generate H2[33].Therefore, the yield of H2is higher than that of H2O2.
It has been proposed that H2O2is mainly derived from the recombination of ·OH, and ·OH is confirmed as the crucial reactive intermediate[5]. As shown in Eqs(1)?(2), h+can react directly with H2O to generate·OH.
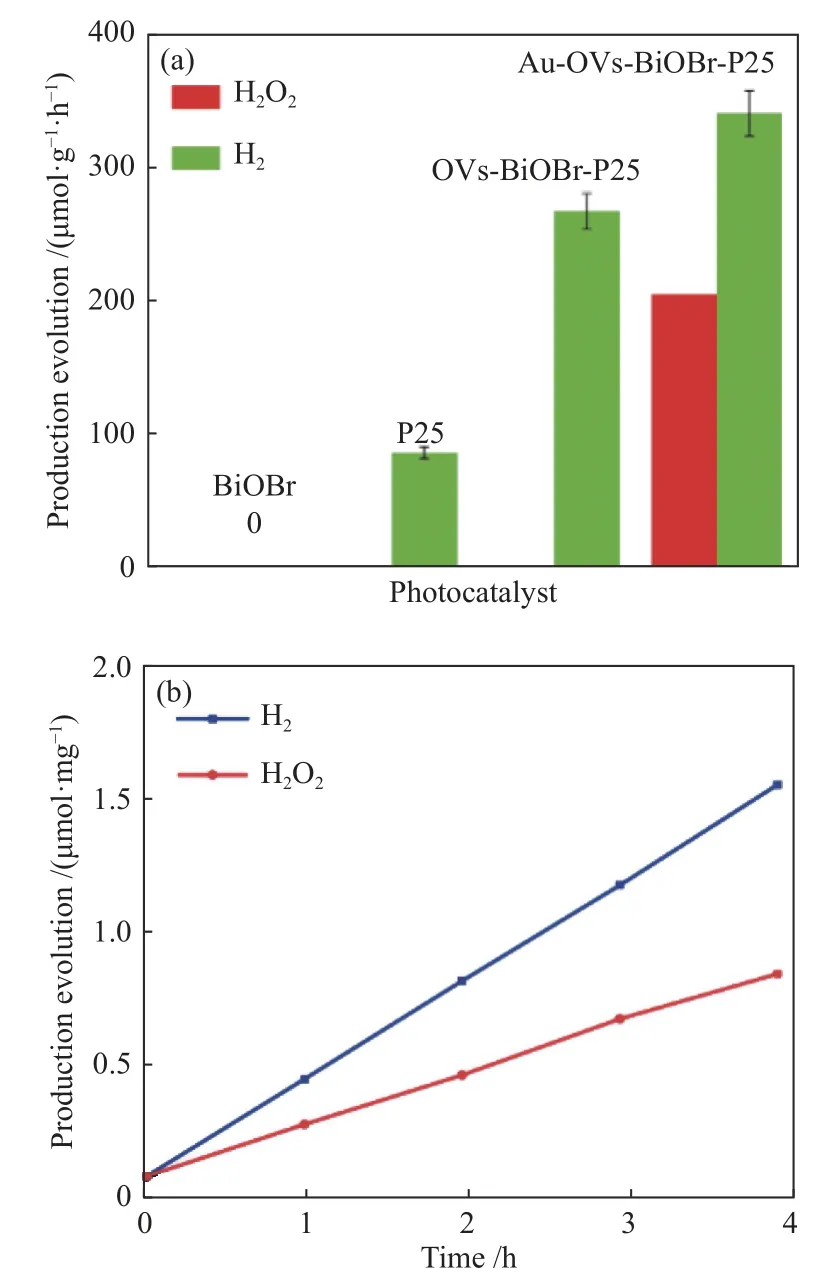
Figure 8 (a) H2 and H2O2 production over BiOBr, P25, OVs-BiOBr-P25 and Au-OVs-BiOBr-P25, (b) H2 and H2O2 production by Au-OVs-BiOBr-P25
The two-electron pathway was supported by ESR.As can be seen in Figure 9, the obvious signal of DMPO, DMPO-OH radical was clearly observed on Au-OVs-BiOBr-P25 after light irradiation,demonstrating the formation of ·OH over Au-OVs-BiOBr-P25. From the above results, it can be seen that overall water splitting is a two-electron transfer process over Au-OVs-BiOBr-P25 (2H2O→H2O2+H2), while OVs-BiOBr-P25 is the four-electron pathway (2H2O→O2+2H2) for overall water splitting[34].
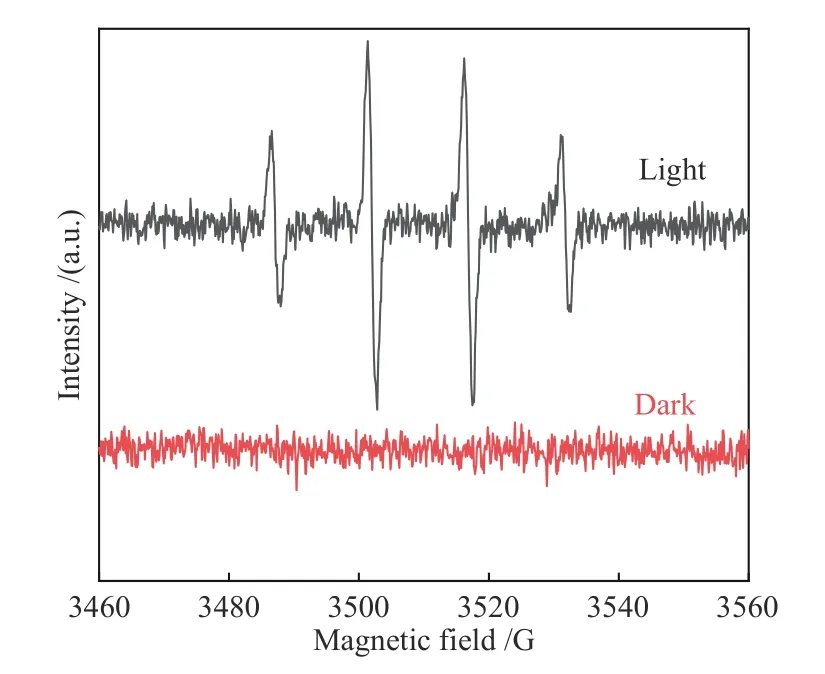
Figure 9 ESR response of DMPO-OH spin adduct in water of Au-OVs-BiOBr-P25 under dark and UV-visible light at N2 atmosphere
2.4 Possible mechanism of photocatalysis
Based on above analysis results, the possible mechanism of photocatalytic overall water splitting over Au-OVs-BiOBr-P25 was proposed, and the scheme graph was illustrated as Figure 10. BiOBr and TiO2form a Z-scheme heterojunction. Under sunlight irradiation, both P25 and BiOBr are excited to form the photoexcited electrons and holes, located in their CB and VB, respectively. Au NPs plays an important role by constructing a bridge for electrons and holes from BiOBr and P25 and the SPR effect of Au NPs can also broaden the visible light adsorption area. Thus, the separation efficiency of photogenerated carriers can be improved. Meanwhile, hydrogen production is carried out on the CB of P25 and the holes in the VB can be used to the oxidation of water on the surface of BiOBr via a two-electron pathway to obtain H2O2.
The enhanced photocatalytic performance of Au-OVs-BiOBr-P25 can be attributed to the following reasons:
The synergetic effects among the SPR of Au NPs,oxygen vacancies and Z-scheme heterojunction in ternary Au-OVs-BiOBr-P25 can enhance the visible light utilization and suppress the recombination of photogenerated electron-hole pairs.
The OVs can provide abundant adsorption sites to promote the water adsorption and accelerate the overall water splitting[35].
Overall water splitting can conduct via a two electron process over noble metal photocatalyst[36].
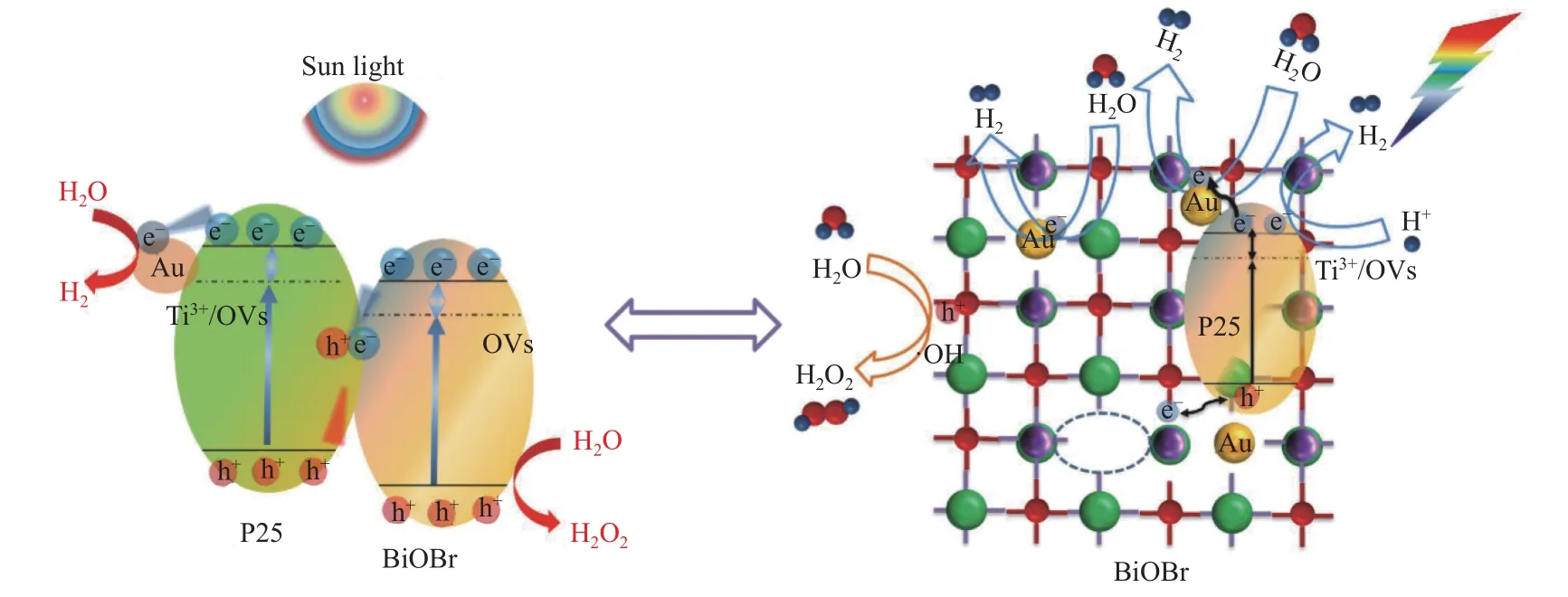
Figure 10 Schematic mechanism of photocatalytic overall water splitting by Au-OVs-BiOBr-P25 in UV-visible light
3 Conclusions
In this study, a novel ternary Au-OVs-BiOBr-P25 Z-scheme photocatalyst was successfully prepared.This ternary system is proposed to be used for photocatalytic overall water splitting. Au-OVs-BiOBr-P25 photocatalysts split water into H2and H2O2via a two-electron pathway(2H2O→H2O2+H2), the formation of heterojunction and the introduction of OVs combined with the SPR effect of Au NPs improve the separation of photogenerated carriers and extend the range of light absorption, thus improving the photocatalytic performance. This work may provide us new ideas for the design of Z-scheme photocatalysts for overall water splitting via the two-electron pathway.
- 燃料化學(xué)學(xué)報(bào)的其它文章
- 鈮元素改性V2O5-WO3/TiO2催化劑降低脫硝過程 SO2 的氧化率
- 甲醇水蒸氣重整制氫Cu-Zn-Al尖晶石催化劑的研究
- Cu3N自支撐電極制備及其電催化氮?dú)膺€原性能研究
- Effects of TiO2 in Pd-TiO2/C for glycerol oxidation in a direct alkaline fuel cell
- Co3O4/WO3復(fù)合催化劑的合成及可見光催化轉(zhuǎn)化甲烷制甲醇
- Solvothermal synthesis of TiO2@MIL-101(Cr) for efficient photocatalytic fuel denitrification

