黃條生長性狀全基因組關聯分析*
崔愛君 徐永江 王 濱 姜 燕 柳學周
崔愛君1,2徐永江1,2①王 濱1姜 燕1柳學周1
(1. 中國水產科學研究院黃海水產研究所 青島海洋科學與技術試點國家實驗室海洋漁業科學與食物產出過程功能實驗室 青島 266071;2. 上海海洋大學水產與生命學院 上海 201306)


全基因組關聯分析(Genome-wide association study, GWAS)是應用全基因組范圍內的大量分子標記(一般為SNP),將標記基因型結合性狀表型進行聯合分析,統計每個標記與目標性狀之間的關聯性大小(一般用值表示),鑒定出與目標性狀密切相關且具有特定功能和育種潛力的基因位點或分子標記,主要用于物種經濟性狀相關SNP分子標記以及功能基因的鑒定,從而達到縮短育種周期和提高育種效率的目的,目前已在畜禽等脊椎動物育種中廣泛應用(Tavares, 2020; Müller, 2019; Cui, 2016; Zhang, 2019)。近年來,隨著基因組高通量測序技術的發展及測序成本的降低,GWAS開始應用于水產養殖動物的育種研究,如在大黃魚()、鯰魚()、凡納濱對蝦()、龍膽石斑魚()、蝦夷扇貝()等物種的生長性狀關聯SNP位點、候選基因的挖掘和鑒定(Zhou, 2019; Li, 2017; Yu, 2019; Wu, 2019; Ning, 2019)方面應用并取得了一定進展。但是,與陸生脊椎動物相比,GWAS在水產動物育種中的應用尚處于起步階段。
1 材料與方法
1.1 實驗材料
1.2 基因組DNA提取與質量檢測
使用QIAGEN公司生產的動物基因組DNA提取試劑盒(DP121221),參照試劑盒使用說明,提取鰭條基因組DNA。用NanoDrop 2000分光光度計(Thermo, 美國)測定基因組DNA濃度,通過1%瓊脂糖凝膠電泳檢測DNA的完整性,通過260 nm/280 nm的比值來判斷DNA的質量。將質檢合格的DNA濃度稀釋至100 ng/μl,于–20℃條件保存備用。
1.3 文庫構建與測序
將≥200 ng的各樣品基因組DNA采用IIB型限制性內切酶XI進行酶切,酶切產物分別加入5組不同的接頭,使用T4 DNA Ligase連接,然后PCR擴增連接產物,最后根據5組接頭信息,將5個標簽按順序串聯,連接產物添加barcode序列,混庫,使用Illumina Hiseq測序平臺對混合好的文庫進行Paired-end測序。
1.4 數據分析

1.4.2 測序數據分析與SNP分型 Illumina HiSeq測序平臺得到的原始圖像數據文件經堿基識別轉化為Raw Reads,過濾刪除含有接頭序列的Reads,得到Clean Reads,過濾刪除含有N堿基比例大于8%的Reads,過濾刪除低質量Reads(質量值低于Q30的堿基超過15%);利用Pear (Zhang, 2014)軟件(V0.9.6)將成對的Clean Reads拼接,提取出各樣品對應的Reads,過濾刪除不含酶切識別位點的Reads后,得到各樣品的Enzyme Reads;利用電子酶切從參考基因組中提取含有酶切識別位點的標簽,作為參考序列,利用SOAP軟件將各樣品的Enzyme Reads比對到參考序列上,主要參數為-r0–M4–v2 (-r0指唯一比對;–M4指最優比對;–v2指比對允許2個錯配),對比對到相同標簽的reads聚類,得到unique標簽深度,選擇樣品深度>3×且深度<500、標簽長度為27 bp的標簽,利用SOAP軟件(V 2.21) (Li, 2008)將測序數據比對到參考序列,利用最大似然法(ML)進行位點的分型(Fu, 2013),過程中使用的RAD分型軟件包(RAD typing),包含10余個軟件組分,覆蓋了從數據預處理至最終分型結果輸出的全過程。
1.4.3 全基因組關聯分析 使用EMMA eXpedited (EMMAX)高效混合模型(Kang, 2010),通過方差分量方法進行SNP分子標記和表型性狀的全基因組關聯分析,所用模型:

式中,為表型值;為固定效應關聯矩陣,為固定效應向量,為通過SNP標記計算得到的關系矩陣,為隨機加性遺傳方差的參數,為剩余效應的向量。
每個SNP位點能得到1個關聯值。對GWAS給出的值劃定2條顯著性水平線,其中1條經Bonferroni校正=0.05/來確定全基因組顯著性閾值(Bonferroni, 1936),為SNP標記的個數,2個性狀經Bonferroni校正后顯著關聯閾值–lg=5.726;另一條使用R軟件包中的p.adjust()函數計算得到經FDR校正后的閾值,體質量性狀潛在顯著關聯閾值–lg= 4.091,全長性狀潛在顯著關聯閾值–lg=4.413,挑選Scaffold長度的前30使用R軟件包的qqman繪制曼哈頓圖,繪制QQ圖對關聯分析進行評價,判斷關聯分析結果是否可靠。
1.5 候選基因鑒定及功能分析


式中,為所有基因中具有KEGG注釋的基因數目,為中差異表達基因中具有的KEGG注釋的基因數目,為所有基因中注釋為某特定KEGG的基因數目,為注釋某特定KEGG的差異表達基因的數目。計算的結果會返回一個富集顯著性的值,小的值表示基因在該Pathway中出現富集,當≤0.05表示顯著富集。
2 結果
2.1 表型性狀描述性統計


Tab.1 Descriptive statistics of growth traits of yellowtail kingfish
2.2 SNP分型
對2b-RAD簡化基因組測序數據按照以下指標進一步過濾。剔除所有樣品中低于80%個體可以分型的位點;剔除MAF低于0.05的位點,剔除等位基因大于2的位點。最終,測序獲得26665個SNP位點進行GWAS分析。
2.3 全基因組關聯分析

圖1 黃條體質量性狀GWAS關聯分析的QQ檢驗
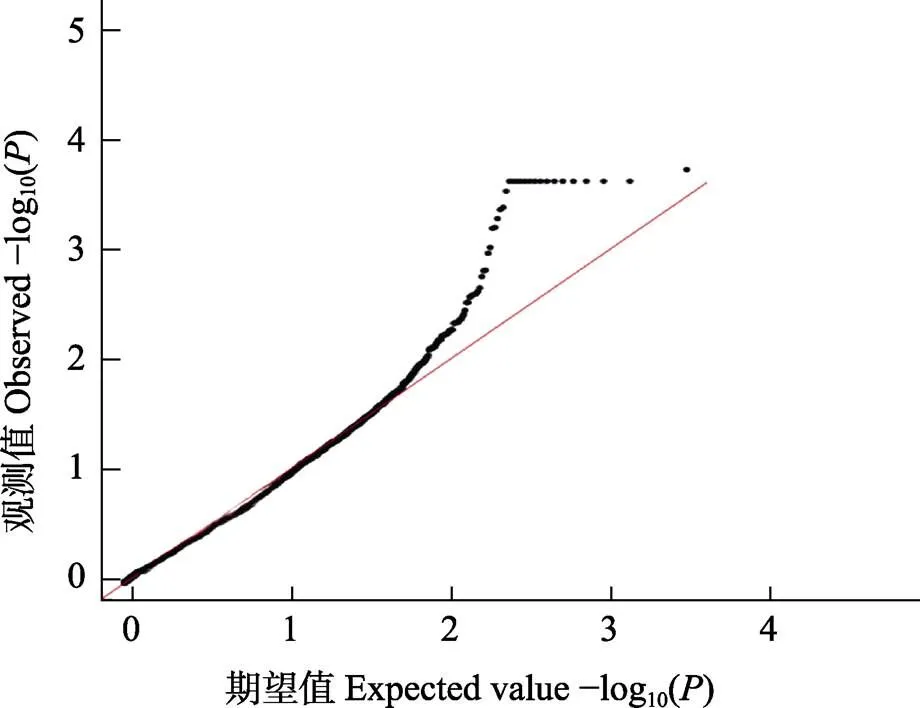
圖2 黃條全長性狀GWAS關聯分析的QQ檢驗

圖3 黃條體質量性狀GWAS分析的曼哈頓圖
紅色實線代表全基因顯著關聯閾值:–log10=5.726, 藍色實線代表潛在顯著關聯閾值: –log10=4.091
The red solid line indicates the genome wide significant threshold: –log10=5.726. The blue solid line indicates the threshold for the significance of “suggestive association”: –log10=4.091
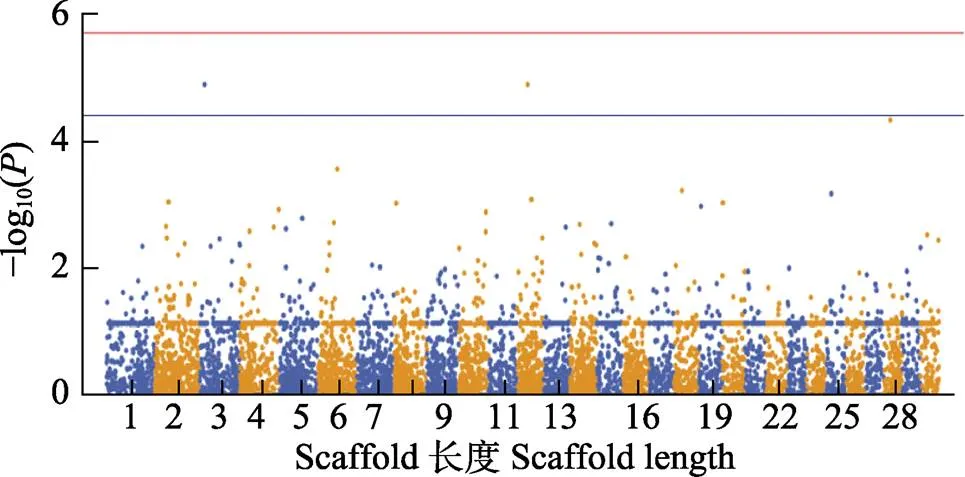
圖4 黃條全長性狀GWAS分析的曼哈頓圖
紅色實線代表全基因顯著關聯閾值–log10=5.726, 藍色實線代表潛在顯著關聯閾值–log10=4.413
The red solid line indicates the genome wide significant threshold: –log10=5.726. The blue solid line indicates the threshold for the significance of “suggestive association”: –log10=4.413
2.4 候選基因生物信息學分析
3 討論

表4 體質量性狀和全長性狀KEGG注釋結果

Tab.4 KEGG annotation results of body weight and total length trait

Bonferroni CE. Teoria statistical delle classi e calcolo delle probabilita. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commericiali di Firenze, 1936, 8: 3–62
Chen SL, Xu WT, Liu Y. Fish genomic research: Decade review and prospect. Journal of Fisheries of China, 2019, 43(1): 1– 14 [陳松林, 徐文騰, 劉洋. 魚類基因組研究十年回顧與展望. 水產學報, 2019, 43(1): 1–14]
Chen ZD, Wang WH. Genome-wide association study on feet weight in chicken (). Journal of Agricultural Biotechnology, 2016, 24(10): 1569–1577 [陳則東, 王文浩. 雞腳重性狀的全基因組關聯分析. 農業生物技術學報, 2016, 24(10): 1569–1577]
Cingolani P, Platts A, Wang LL,. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome ofstrain w1118; iso-2; iso-3. Fly, 2012, 6(2): 80–92
Cui ZH, Luo JD, Qi CY,Genome-wide association study (GWAS) reveals the genetic architecture of four husk traits in maize. BMC Genomics, 2016, 17(1): 946
Fu X, Dou J, Mao J,RAD typing: An integrated package for accuratecodominant and dominant RAD genotyping in mapping populations. PLoS One, 2013, 8(11): e79960
Johannessen M, Moller S, Hansen T,. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cellular and Molecular Life Sciences, 2006, 63(3): 268–284
Kang HM, Sul JH, Service SK,. Variance component model to account for sample structure in genome-wide association studies. Nature Genetics, 2010, 42(4): 348–354
Li N, Zhou T, Geng X,. Identification of novel genes significantly affecting growth in catfish through GWAS analysis. Molecular Genetics and Genomics, 2017, 293(3): 1–13
Li R, Li Y, Kristiansen K,. SOAP: Short oligonucleotide alignment program. Bioinformatics, 2008, 24(5): 713–714


Matthias C, Edwige T, Bernadette B,. FHL2 interacts with both ADAM-17 and the cytoskeleton and regulates ADAM- 17 localization and activity. Journal of Cellular Physiology, 2006, 208: 363–372
Müller BSF, de Almeida Filho JE, Lima BM,. Independent and joint-GWAS for growth traits inby assembling genome-wide data for 3373 individuals across four breeding populations. New Phytologist, 2019, 221(2): 818–833
Naha BC, Prasad A, Sailo L,, Concept of genome wide association studies and its progress in livestock. International Journal of Science and Nature, 2016, 7(1): 39–42
Nguyen NH, Premachandra HKA, Kilian A,. Genomic prediction using DArT-Seq technology for yellowtail kingfish. BMC Genomics, 2018a, 19(1): 107
Nguyen NH, Rastas PMA, Premachandra HKA,. First high- density linkage map and single nucleotide polymorphisms significantly associated with traits of economic importance in yellowtail kingfish. Frontiers in Genetics, 2018b, 9: 127
Ning XH, Li X, Wang J,. Genome-wide association study reveals E2F3 as a candidate gene for scallop growth. Aquaculture, 2019, 73(4): 734216
Ohara E, Nishimura T, Nagakura Y,Genetic linkage maps of two yellowtails (and). Aquaculture, 2005, 244: 41–48
Pi X, Ren R, Kelley R,Sequential roles for myosin-X in BMP6 dependent filopodial extension, migration, and activation of BMP receptors. Journal of Cell Biology, 2008, 179(7): 1569–1582
Premachandra HKA, De la Cruz FL, Takeuchi Y,. Genomic DNA variation confirmedcomprises three different populations in the Pacific, but with recent divergence. Scientific Reports, 2017, 7(1): 9386
Raise A, Stefanie W, Ralf J,. Hunting for the function of orphan GPCRs-beyond the search for the endogenous ligand. British Journal of Pharmacology, 2015, 172(13): 3218–3228
Sepulveda FA, Gonzalez M. Spatio-temporal patterns of genetic variations in populations of yellowtail kingfishfrom the southeastern Pacific Ocean and potential implications for its fishery management. Journal of Fish Biology,2017, 90(1): 249–264

Sicuro B, Luzzana U. The state ofspp. other than yellowtail () farming in the world. Reviews in Fisheries Science and Aquaculture, 2016, 24(4): 314–325
Swart BL, Merwe BVD, Kerwath SE,Phylogeography of the pelagic fishat different scales: Confirmationof inter-ocean population structure and evaluation of southern African genetic diversity. South African Journal of Marine Science, 2016, 38(4): 513–524
Symonds JE, Walker SP, Pether S,. Developing yellowtail kingfish () and hāpuku () for New Zealand aquaculture. New Zealand Journal of Marine and Freshwater Research, 2014, 48(3): 371–384
Tao L, He XY, Di R,. Research progress on genome-wide association study for growth-related traits in livestock and poultry. Chinese Journal of Animal Science, 2019, 55(11): 34–41 [陶林, 賀小云, 荻冉, 等. 畜禽生長發育相關性狀的全基因組關聯分析研究進展. 中國畜牧雜志, 2019, 55(11): 34–41]
Tavares V, Pinto R, Assis J,. Venous thromboembolism GWAS reported genetic makeup and the hallmarks of cancer: Linkage to ovarian tumour behavior. Biochimica et Biophysica Acta - Reviews on Cancer, 2020, 1873(1): 188331
Wang B, Xu Y, Liu X, et al. Molecular characterization and expression profiles of insulin-like growth factors in yellowtail kingfish () during embryonic development. Fish Physiology and Biochemistry, 2019, 45(1): 375-390
Whatmore P, Nguyen NH, Miller A,. Genetic parameters for economically important traits in yellowtail kingfish. Aquaculture, 2013, 400(25): 77–84
Woolner S, O'Brien LL, Wiese C, etMyosin-10 and actin filaments are essential for mitotic spindle function. Journal of Cell Biology, 2008, 182(1): 77–88
Wu LN, Yang Y, Li, BJ,. First genome-wide association analysis for growth traits in the largest coral reef-dwelling bony fishes, the giant grouper (. Marine Biotechnology, 2019, 21(5): 707–717
Yu Y, Wang QC, Zhang Q, Genome scan for genomic regions and genes associated with growth trait in pacific white shrimpMarine Biotechnology, 2019, 21(3): 374–383
Zhang J, Kobert K, Flouri T,. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics, 2014, 30(5): 614–620
Zhang YF, Zhang JJ, Gong HF,. Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on GWAS data in six pig populations. Meat Science, 2019, 150: 47–55
Zhou, Z. Han, K. Wu, Y,Genome-wide association study of growth and body-shape-related traits in large yellow croaker () using ddRAD sequencing. Marine Biotechnology, 2019, 21(5): 655–670
Genome-Wide Association Analysis of Growth Traits in Yellowtail Kingfish ()
CUI Aijun1,2, XU Yongjiang1,2①, WANG Bin1, JIANG Yan1, LIU Xuezhou1
(1.Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266071; 2. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306)
The genetic resources available for the commercially important pelagic yellowtail kingfish () are relative sparse. 2b-RAD simplified genome sequencing technology was applied to screen single nucleotide polymorphisms (SNPs) in yellowtail kingfish, and a total of 26,665 SNPs were obtained. A genome-wide association study was carried out to detect body weight- and total length-associated SNPs in 119 individuals from the yellowtail kingfish population in the Yellow Sea. The results showed that 17 SNPs associated with body weight and with potential genome-wide significance were found. Genes in the candidate regions with 1 Mb windows were screened, and 17 candidate genes were obtained. A total of 12 SNPs associated with total length and with potential genome-wide significance were identified, and 12 candidate genes were found. For these candidate genes, KEGG pathway analysis showed that they are mainly involved in the metabolic regulation pathway of growth and development in other vertebrates, which may be important candidate SNP loci and functional genes closely related to the growth traits of yellowtail kingfish. The present results could provide genetic information for the sustainable utilization of germplasm resources and genetic breeding of yellowtail kingfish in the in the future.
; Growth trait; Genome-wide association study (GWAS); 2b-RAD; Simplified genome
XU Yongjiang, E-mail: xuyj@ysfri.ac.cn
S917; Q78
A
2095-9869(2021)02-0071-08
10.19663/j.issn2095-9869.20200205002
http://www.yykxjz.cn/

Cui AJ, Xu YJ, Wang B, Jiang Y, Liu XZ. Genome-wide association analysis of growth traits in yellowtail kingfish (). Progress in Fishery Sciences, 2021, 42(2): 71–78
*山東省支持青島海洋科學與技術試點國家實驗室重大科技專項(2018SDKJ0303-1)、中國水產科學研究院基本科研業務費(2019GH15)、山東省重點研發計劃項目(2018GHY115044)、國家重點研發計劃項目(2019YFD0900901; 2018YFD0901204)和國家海水魚產業技術體系(CARS-47)共同資助 [This work was supported by Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018SDKJ0303-1), Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2019GH15), Key Research and Development Program of Shandong Province (2018GHY115044), National Key Research and Development Program of China (2019YFD0900901; 2018YFD0901204) and China Agriculture Research System (CARS-47)]. 崔愛君,E-mail: aijun0218@126.com
徐永江,研究員,E-mail: xuyj@ysfri.ac.cn
2020-02-05,
2020-03-02
(編輯 馮小花)

