Fabrication of Z-scheme BiVO4/GO/g-C3N4 Photocatalyst with Efficient Visble-light Photocatalytic Performance
XU Shichao, ZHU Tianzhe, QIAO Yang, BAI Xuejian, TANG Nan, ZHENG Chunming
Fabrication of Z-scheme BiVO4/GO/g-C3N4Photocatalyst with Efficient Visble-light Photocatalytic Performance
XU Shichao1, ZHU Tianzhe1, QIAO Yang2, BAI Xuejian2, TANG Nan1, ZHENG Chunming2
(1. School of Environmental Science and Engineering, Tiangong University, Tianjin 300389, China; 2. School of Chemistry and Chemical Engineering, Tiangong University, Tianjin 300389, China)
Fabricating Z-scheme photocatalysts is a promising method for improving photocatalytic activity by effectively enhancing charge separation. A new Z-scheme BiVO4/GO/g-C3N4photocatalyst was prepared by two steps of impregnation-calcination and hydrothermal method, and then characterized by different methods. In the photocatalytic process of BiVO4/GO/g-C3N4, GO nanosheet act as fast transmission channels between BiVO4and g-C3N4and can suppress electron-hole recombination, which significantly promotes the charge separation and improves the redox ability of the ternary heterojunction. The ternary photocatalyst has good photocatalytic degradation of Rhodamine B (RhB) as compared to the single-component or binary composite. It is capable of degrading 85% of RhB in 120 min under visible light irradiation and the hole (h+) plays a major role in the reaction. This work provides a simple preparation method for a ternary photocatalyst system in which g-C3N4coupled with BiVO4by GO to significantly improve photocatalytic activity.
BiVO4; g-C3N4; GO; ternary photocatalyst; Z-scheme heterojunction
In recent decades, organic dyes have been widely used in many industrial production process, such as clothing, food processing, medicine, spray and rubber manufactur-ing industries[1-2]. The emissions of organic dye wastewater from these industries are growing and become a challenge to human. Photocatalytic decomposi-tion of organic dyes in aquatic environment on semiconductor catalysts, such as TiO2[3], WO3[4], ZnO[5], MoS2[6-7], is a potential technique to tackle the organic pollution. However, the wide band gap and poor quantum yield of these traditional semiconductor catalysts significantly limit their efficient absorption of visible-light, which is essential for photocatalysts, and result in low photocatalytic efficiency.
Graphitic carbon nitride (g-C3N4), a non-metal pol-y---meric visible-light driven photocatalyst with suitable band gap (around 2.7 eV) and valence band (around 1.5 eV) has received intense interest in photocatalysis community owing to its excellent chemical stability, relatively low cost and non-toxicity[8-9]. Nevertheless, pure g-C3N4faces high recombination rate of photogenerated carriers, inadequate visible-light absorption range and small specific surface area[10]. Tremendous attempts have been made to make up for these shortcomings, and a Z-scheme photocatalytic system would be a better approach to tackle the problems. An electron mediator or redox shuttle was applied in Z-scheme to regulate charge transfer, creating spatial isolation of photogenerated carriers, more positive valence band potential and more negative conduction band[11]. The system can inhi-bit the recombination of electrons-holes pairs, and subse-quently improve visible- light induced photocatalytic per-formance. Recently, many Z-scheme photocatalysts have been prepared to degrade hazardous organic compounds, such as g-C3N4/TiO2[12], MoO3/g-C3N4[13], g-C3N4/ZnO[14], SnO2/Zn2SnO4[15].
Bismuth vanadate (BiVO4) possesses relatively narrow bandgap (~2.43 eV) and valence band (~2.75 eV), high chemical stability and remarkable energy conversion, and can act as a photocatalyst to degrade organic pollutants[16-17]. However, for pure BiVO4, there are pro-b-lems such as large particle size, small specific surface area, low visible light absorption, and high electron-hole recombination rate, which subsequently leads to low pho-tocatalytic activity[18]. Therefore, it is necessary to select suitable semiconductor and recombine BiVO4into a Z- scheme heterojunction, which could promote the separation of photogenerated carriers to improve the pho-t-oca-talytic efficiency. Moreover, GO (graphene oxide) owns outstanding electrical conductivity, excellent mechanical properties and high surface area, and could be applied in Z-scheme system as a cocatalyst to improve electron transfer rates and photocatalytic activity[11,19].
In this work, a new photocatalyst with a ternary heterojunction, BiVO4/GO/g-C3N4, was prepared by a two- step method. The binary composite GO/g-C3N4was synthesized by impregnation-calcination method and combined with BiVO4to form a ternary heterojunction. The photocatalytic performance of the ternary system (BiVO4/ GO/g-C3N4), the binary system (GO/g-C3N4) and single systems (g-C3N4and BiVO4) under visible-light radiation, was systematically evaluated by Rhdamine B degradation, and the mechanism of photocatalytic reaction of the Z-scheme heterojunction BiVO4/GO/g-C3N4was discu-ssed in detail.
1 Experimental
1.1 Materials and synthesis
Dicyandiamide, bismuthnitrate pentahydrate (Bi(NO3)3·5H2O), ammonium metavanadate (NH4VO3), were purchased from Aladdin Chemical Reagent Co. Ltd (Shanghai, China). Graphene oxide (GO) powder was purchased from Suzhou Hengqiu Graphene Co., Ltd., China.
1.1.1 Preparation of the GO/g-C3N4
GO/g-C3N4binary photocatalytic heterojunction was prepared by an impregnation-calcination method according to previous report with some modifications[20]. The GO/g-C3N4samples were synthesized as follows: 36 mg GO was put into 300 mL water, stirred and ultrasound for 0.5 h. Then 120 mg dicyandiamide was dispersed in mixture solution. The mixture solution was ceaselessly stirred at 80 ℃ for 4 h, and then dried in an oven at 80 ℃ for 8 h. In this process, dicyandiamide could deposit on the GO surfaceelectrostatic interactions. The as-prepared gray solid samples was put into crucibles with the covers, and then heated to 520 ℃ for 2.5 h at a heating rate of 5 ℃·min–1and kept at 520 ℃ for 2 h in a quartz tube under flowing pure argon. The calcined product was ground to powder, washed with deionized water for three times and then dried at 60 ℃. The ultimate pale-yellowish green GO/g-C3N4powder was prepared.
1.1.2 Preparation of the BiVO4/GO/g-C3N4ternary photocatalyst
Firstly, 0.7 mmol Bi(NO3)3·5H2O and 0.7 mmol NH4VO4were completely dissolved in 10 mL 2 mol/L HNO3aqu-e-ous solution and 10 mL deionized water, respectively. Secondly, different amounts of GO/g-C3N4were added to Bi(NO3)3mixture solution and dispersed by 0.5 h stirring and ultrasound, then NH4VO4aqueous solution was put into the above mixture solution. In order to get the precursor, the resulting pale yellow suspension was vigorous stirred for 1 h at room temperature. Then it was poured into a 50 mL Teflon-lined autoclave, heated at 180 ℃ for 7 h, and naturally cooled to room temperature. The obtained precipitate was washed with deionized water and absolute ethanol for 3 times, respectively. Finally, the samples were dried at 60 ℃ for 4 h. The composite photocatalysts having different mass ratios of BiVO4to GO/g-C3N4were recorded as 8 : 2, 6 : 4, 4 : 6 and 2 : 8 (the former is BiVO4occupancy). Here the photocatalytic heterojunctions were denoted as B8GC2, B6GC4, B4GC6, B2GC8, respectively according to these different mass ratios. Meanwhile, for comparison, pure BiVO4was also prepared in the same manner without the addition of GO/g-C3N4.
1.2 Characterizations
X-ray diffraction (XRD) patterns were characterized on a D/Max-2500 X-ray diffractometer (Rigaku, Japan) with Cu-Kα radiation at a scan rate (2) of 0.05 (°)/s in the range of 3°–70°. The microstructures were examined by a transmission electron microscope (TEM) (Hitachi H7650, HITACHI, Japan) and high-resolution transmission electron micrographs (HRTEM) (TecnaiG2 F20, FEI,America). The sizes and morphologies were collected with a field emission scanning electron microscope (Gemini SEM500, ZEISS, Germany). UV-visible absorb-ance spe-c-tra of the dry-pressed disk samples were obtained by means of a UV-visible spectrophotometer (UV-2600, Sh-i-madzu, Japan) in the range of 200–800 nm with BaSO4as a reflectance standard. The chemical bonding status of the samples were analyzed on an FT-IR spectro-meter (FTIR-650, Gangdong Technology, China). The specific surface area of the samples were measured through a mesoporous surface physical adsorber (NOVA4200e, Quantachrome, America). Photoluminescence (PL) spectra were measured under 315 nm excitation wavelength by a Fluorescence Spectrophotometer (F380, Gangdong Technology, China).
1.3 Photoelectrochemical measurements
All photoelectrochemical studies were carried out by an electrochemical workstation (CHI760E, Chenhua, China) in a standard three-electrode system which used the as- prepared samples as the working electrodes, the platinum wire as the counter electrode, and Ag/AgCl elec-trode as the reference electrode. 0.1 mol/L Na2SO4aqueous solution was used as the electrolyte. The working electrodes were prepared as follows: 0.2 g photocatalyst was ground and 0.5 mL anhydrous ethanol with the ultrasonic dispersion to make uniform suspension slurry. Then, the obtained slurry was smeared onto a 2 cm×2 cm F-doped SnO2- coated conducting glass (FTO glass) using spincoating strategy for twice. Photocurrent curves were measured using an Amperometric-curves method under a 30 s intermittent irradiation, and the initial voltage was 1.2 V. Electrochemical impedance spectra (EIS) were obtained in the frequency range of 0.01–100,000 Hz at initial voltage of 0 V.
1.4 Evaluation of photocatalytic activity
A 500 W Xe lamp (Beijing AuLight, China) was utilized as a visible-light source with 400 nm cut-off filters. The degradation of RhB was used as the evaluation standard for the photocatalytic ability of the sample. The photocatalytic degradation products of RhB were analyzed using an UV-Vis spectrophotometer (UV-2600, SHIMADZU, Japan) at 553 nm.
In the photocatalytic performance evaluation experiment, 10 mg catalysts was added into the RhB (20 mL, 20 mg·L–1) each time. In order to reach adsorption- desorption equilibrium, the solution was stirred for 30 min in the dark before the irradiation. Suspensions with dosage of 4.0 mL were withdrawn and analyzed regularly. After suspensions were centrifuged (8000 r/min, 10 min) for photocatalyst removal, detected at 553 nm absorbance by UV-Vis spectrophotometer.
The photocatalytic degradation efficiency () was obtained for measurement and calculation according to Eq. (1):
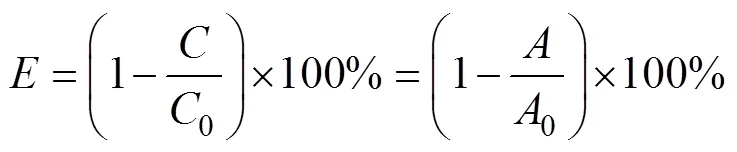
Whereis the concentration of RhB at different time,0is the initial concentration at the RhB adsorption equilibrium,and0are the corresponding absorption values.
2 Results and discussion
2.1 Optical, structural and morphological characteristics
The reflective patterns of as-prepared composite samples were studied by powder XRD to infer the phase structures. As shown in Fig. 1(a), the strongest diffraction (002) peak near 2=27.4° corresponds to an interplanar distance of the conjugated aromatic system of 0.326 nm, which reflects the interlayer tight accumulation of aromatic rings. Another apparent additional (001) peak relates to an in-plane structural packing motif which interlayer space is 0.672 nm[21]. In addition, due to incomplete pyrolysis of reactant, the weak diffraction peaks of intermediates were observed in the XRD patterns.
In addition, compared to the crystalline structures of pure BiVO4, no matter whether it is the shape or position, the added compound has little effect on the characteristic peak of pure BiVO4. Therefore, BiVO4in heterojunction maintains a stable crystal structure, which isn’t affected by the introduction of GO/g-C3N4. Due to no introduction of other new peaks, the combination of BiVO4and GO/g-C3N4don’t generate impurities, revealing that GO, g-C3N4and BiVO4coexist in the ternary nanocomposite photocatalysts.
FT-IR spectra (Fig. 1(b)) show that graphite oxide exhi-bits strong hydroxyl stretching vibration absorption peak near 3477 cm?1. The C=O stretching vibration peak onthe carboxyl group of graphene oxide appears at 1790 cm–1. The absorption peak at 1685 cm–1may be the absorption peak due to the bending vibration of C–OH[22]. The peak at 1116 cm–1derives from the vibration of graphene oxide C–O–C. Therefore under the experimental conditions, at least 4 functional groups of –OH, –COOH, C–O–C and –C=O exist in the graphite oxide[23]. The infrared spectrum of GO/g-C3N4shows that the region of 1200– 1650 cm–1contains several strong peaks with peaks at 1232, 1326, 1415, 1567 and 1642 cm–1, which are typical CN heterocycles. The characteristic peak caused by tensile vibration and the peak at 806 cm–1is the characteristic breathing pattern of the triazine unit. No other peaks were observed in BiVO4/GO/g-C3N4spectrum, probably due to the reduction of graphene oxide during the calcination. Vibration band at 739 cm–1is generated by(VO43–) stretching vibration[24]. The part of primary bands of g-C3N4and BiVO4are included in the ternary nanocomposite, indicating that the BiVO4/ GO/g-C3N4ternary photocatalyst was synthesized successfully.

Fig. 1 XRD patterns of BiVO4, g-C3N4/GO, B2GC8, and BiVO4/ GO/g-C3N4 (a), and FT-IR spectra of the as-prepared GO, GO/ g-C3N4, BiVO4, BiVO4/GO/g-C3N4 (b)
To examine chemical composition and states of samples, the prepared photocatalysts were analyzed by XPS characterization (Fig. 2). It is obvious that the detection spectrum of the BiVO4/GO/g-C3N4can determine that the sample contains only Bi, V and O elements for BiVO4, and C, N and O elements for GO/g-C3N4, and no impurities (Fig. 2(a)). According to the Gaussian curve- fitted signal deconvolution, the high-resolution N1s spectrum of BiVO4/GO/g-C3N4has N species in different chemical environments (Fig. 2(b)). The highest N1s peak at 398.6 eV corresponds to sp2-hybridized nitrogen (C=N–C). Two other weak N1s peaks locating at 399.8and 401.5 eV can be assigned to tertiary nitrogen (N–(C)3) and amino functional groups (C–N–H), respectively. The weak C1s peak (284.6 eV) in the BiVO4sample spectrum was produced by the XPS instrument itself contaminating hydrocarbons, whereas the peak of the GO/g-C3N4sample at 288.08 eV was attributed to the sp2-hybridized carbon in N-containing aromatic ring (N–C=N), which positively shifted to 288.38 eV for the BiVO4/GO/g-C3N4sample in Fig. 2(c)[25]. The Bi4f spectrum (Fig. 2(d)) of pristine BiVO4at 159.08 and 164.48 eV are respectively corresponding to Bi4f7/2 and Bi4f5/2 of Bi3+induced, while these two peaks in the spectrum of BiVO4/GO/g- C3N4shifted to 158.98 and 164.38 eV, respectively. Com-pared to Bi4f of pure BiVO4, B2GC8 exhibits a weak blue shift effect, demonstrating that GO/g-C3N4acts as an electron acceptor and promotes the carrier separation.
TEM images (Fig. 3(a, b) show that the layered g-C3N4tightly anchors on the wrinkled surface of the GO to form a stable heterojunction in the GO/g-C3N4composite. After hydrothermal reaction, 1–5 μm BiVO4crystal particles uniformly distributed on the surface of GO/g-C3N4, and a ternary photocatalyst was synthesized. In Fig. 3(c), the pure BiVO4crystal in the shape of decagon and polyhedron is monoclinic, which is consistent with the results of XRD. It can be observed that the pure BiVO4particles own average size of 1–5 μm, while due to the introduction of GO/g-C3N4in the system, BiVO4crystals become more delicate and reduce to the size of 100– 500 nm (Fig. 3(d)), which may be affected by the chem-ical environment on the surface or edge of GO/g-C3N4, such as hydroxyl and carbonyl that could easily limit the nucleation and growth process of the BiVO4crystal. The layered structure of GO/g-C3N4may include stacking layers, which provides a site for the production and growth of BiVO4particles, and are in accordance with the XRD results.
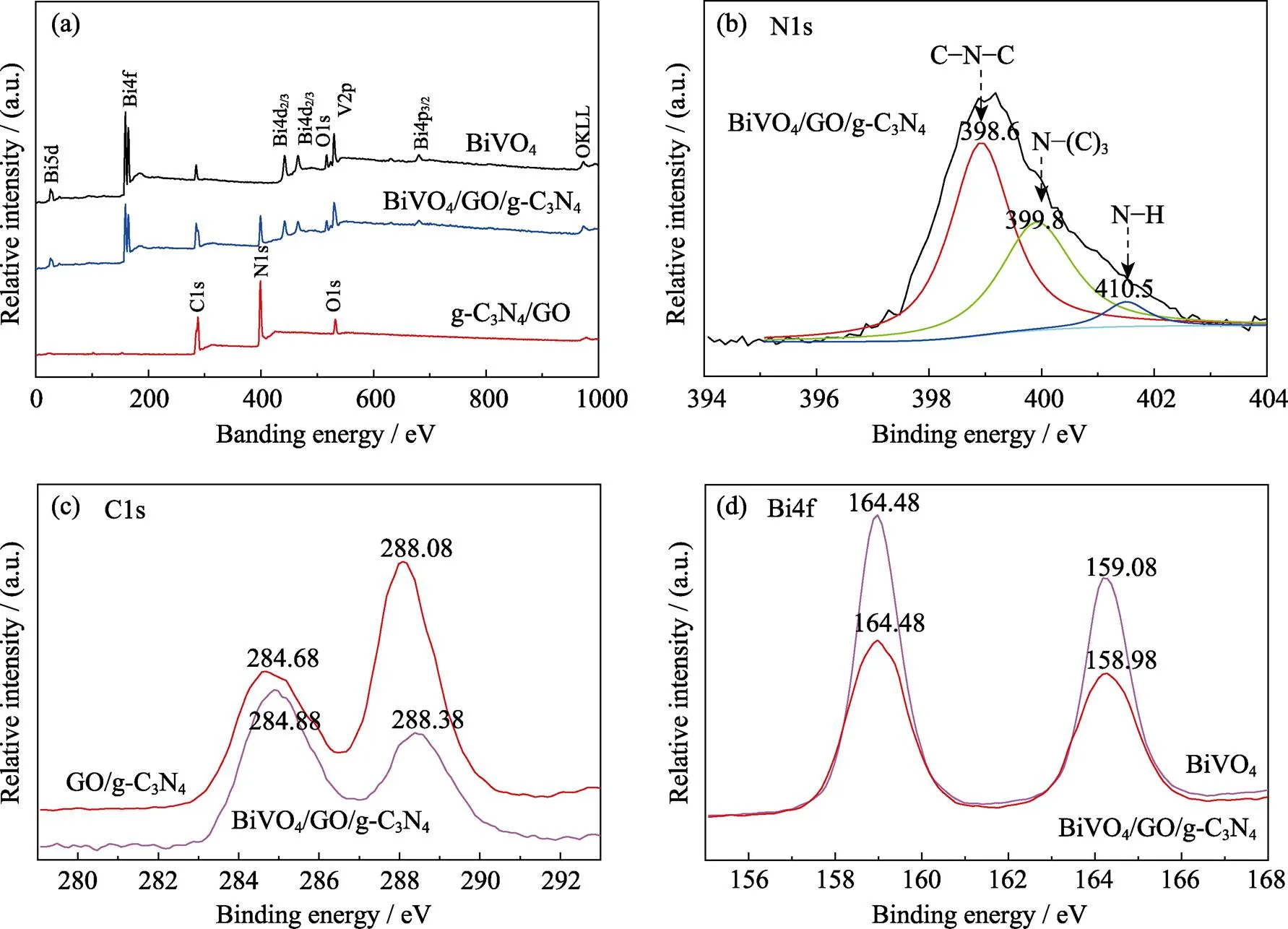
Fig. 2 XPS survey scans of BiVO4, GO/g-C3N4 and BiVO4/ GO/g-C3N4(a), and high-resolution spectra of N1s (b), C1s (c), Bi1s (d) in BiVO4, GO/g-C3N4 and BiVO4/GO/g-C3N4, respectively

Fig. 3 TEM images of the as-prepared GO/g-C3N4 (a) and BiVO4/GO/g-C3N4(b), and SEM images of BiVO4 (c) and BiVO4/GO/g-C3N4 (d)
The optical property of semiconductor photocatalysts plays a significant role in investigating the energy band structure for the photocatalytic activity. The UV-Vis diffuse reflectance spectra (DRS) of the obtained samples were revealed in Fig. 4(a). The absorption edge of the as-synthesized pure g-C3N4is at about 556 nm, while a broader absorption edge is at about 550 nm, suggesting that BiVO4can effectively utilize visible light for photocatalysis. The addition of GO to the GO/g-C3N4composite increases the background absorption of the sample, allowing the sample to more fully utilize visible-light as compared with pure g-C3N4. Moreover, B2GC8 exhibits similar absorption characteristics to pure BiVO4, indicating that the binding of GO/g-C3N4only on the surface of BiVO4did not change the original lattice structure, which is consistent with XRD and SEM results. The widest visible light absorption of the ternary BiVO4/GO/ g-C3N4composites show that BiVO4, g-C3N4and GO generate effective synergy.
The band gaps were elevated by the following Eq. (2):
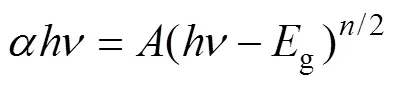
Where,,, andare absorption coefficient, Planck constant, light frequency, and a constant, respectively. Thevalue is determined by the type of optical transition of semiconductors (=1 for direct transition and=4 for indirect transition). BiVO4belongs to the direct transition semiconductors but g-C3N4direct transition semiconductors. Through data processing and analysis, thegvalues of BiVO4(2.43 eV) and g-C3N4(2.7 eV) can be obtained respectively (Fig. 4(b)).
2.2 Photocatalytic activity analyses
The visible light photocatalytic degradation spectra of the RhB with obtained photocatalysts is showed in Fig. 5(a). It is apparent that g-C3N4and BiVO4exhibit poor photocatalytic degradation of RhB solution activity under visible light owing to faster photogenerated electron/hole pair recombination and relatively narrow absorption of visible light regions, which is the same as the UV-Vis diffuse reflectance spectra.
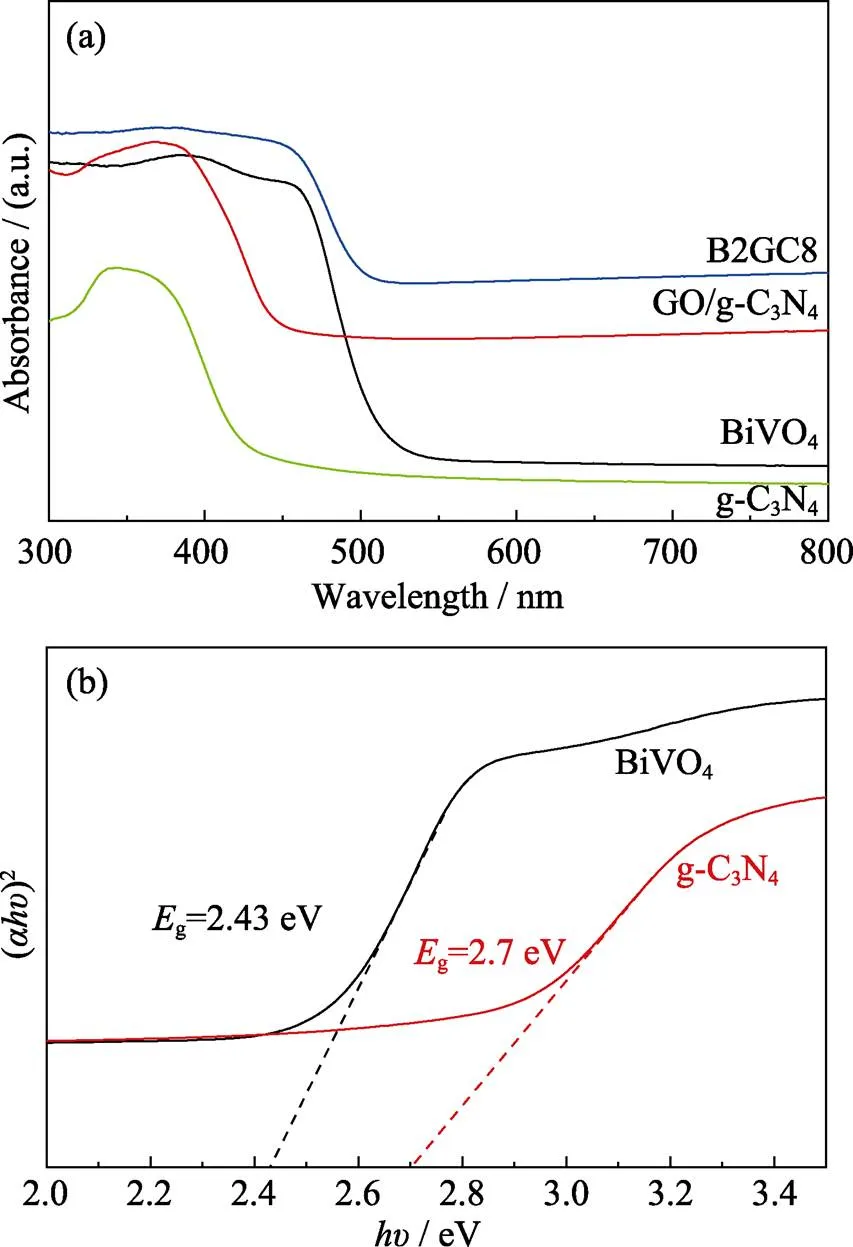
Fig. 4 UV-Vis adsorption spectra of prepared samples (a) and calculated band gap of BiVO4 and g-C3N4 (b)
Although g-C3N4and BiVO4respond to visible light, the effect is not significant, and the degradation efficiencies after irradiation for 120 min under visible light are 17% and 22%. As contrasted with g-C3N4and BiVO4, the photocatalytic degradation efficiency of RhB by GO/g- C3N4and B2GC8 composites is significantly improved, reaching 51% and 85%, respectively. The specific surface areas of g-C3N4,BiVO4, GO/g-C3N4and B2CG8 are 7.6, 1.7, 5.7, 3.2 m2·g–1, respectively. The calculated reaction rates per unit area were 0.197, 1.17, 0.68, 3.29 mg·h–1·m–2, respectively. The degradation efficiency of the ternary BiVO4/GO/g-C3N4composite is higher than those of other catalysts, and the photoluminescence (PL) intensity of the ternary heterojunction was very low. The above results indicate that the Z scheme ternary photocatalyst has an effective redox capability and an excellent charge separation driving force to degrade RhB. The comparison results show that the Z-scheme ternary heterojunction has significant photocatalytic activity under visible light in terms of degradation of RhB.
Fig. 5(b) shows that the photocatalytic decomposition of RhB is supposed to follow a pseudo-first-order kinetics reaction, and the evaluating formula is expressed as Eq. (3):
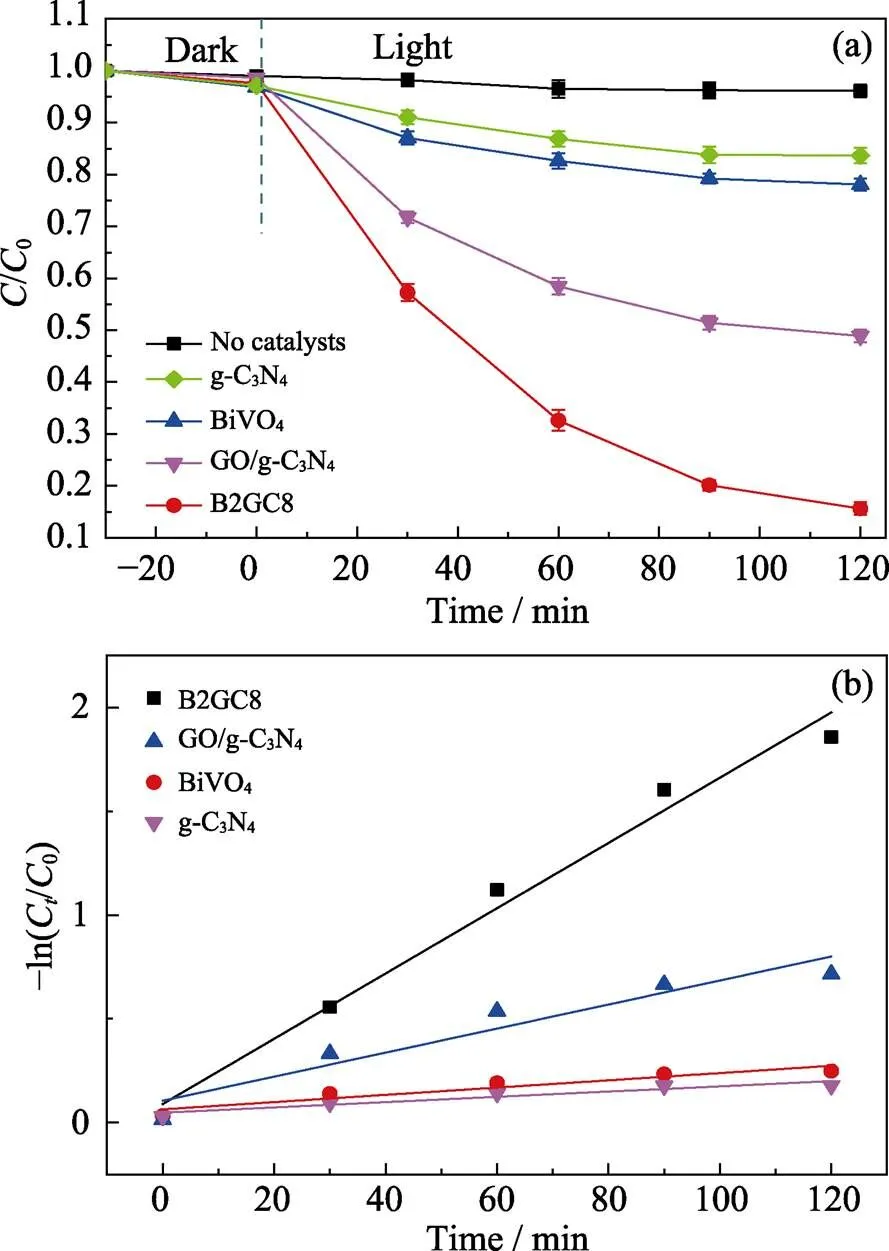
Fig. 5 Photocatalytic degradation of RhB (a) and dynamics of RhB photodegradation reaction (ln(C0/C) (b)
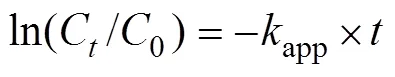
Whereappis the apparent rate constant (min?1),0andCis the RhB concentration at reaction time 0 and, respectively.
2.3 Photoelectrochemical analyses
In order to obtain more evidence that Z-scheme heterojunction promotes photocatalytic degradation of dyes, samples electrodes are recorded for several on-off cycles of irradiation. As can be seen from Fig. 6(a), thecurve of the synthetic sample was analyzed by a 30 s interval dark and visible light illumination cycle. When visible light is irradiated, the photocurrent value is gradually stabilized, and when the light is turned off, the photocurrent rapidly drops to zero, and this process can be cyclically reproduced. The performance indicates that the generated electrons move in the direction of the visible light, thereby generating a certain intensity of photocurrent. In the present case, the photocurrent diagram of B2GC8 ternary heterojunction and GO/g-C3N4binary composites reveals an improved photocurrent response than that of the single BiVO4, which indicates excellent charge separation efficiency. This apparent enhancement of photocurrent also demonstrates that due to spatial isolation of photogenerated carriers at the interface between BiVO4, GO and g-C3N4, photogenerated electron-hole pairs achieves lower recombination and more efficient separation for the GO/g-C3N4composites. The reduced species in the electrolyte are trapped or captured the hole on the BiVO4surface, while the electrons are effectively transported to g-C3N4by GO sheets.
Photoluminescence (PL) analysis was carried out to reveal the intensity of the fluorescence emitted by the recombination of photogenerated electrons and the holes, thus inferring the process of migration, separation and recombination of photogenerated electrons and holes in catalyst. It is obvious that at an excitation wavelength of 315 nm, BiVO4is excited to a very strong steady-state fluorescence intensity, and the GO/g-C3N4composite is weaker than that of BiVO4, which is attributed to the fact that GO can act as an electronic repository to facilitate charge transfer and inhibit charge recombination in Fig. 6(b). The B2GC8 composite was excited to have the weakest fluorescence intensity, indicating that it has a lowest recombination rate of photogenerated carriers under visible-light irradiation. This can be mainly attributed to the transfer of electrons excited on BiVO4to the valence band of g-C3N4through GO, while the holes on g-C3N4are transferred to the conduction band of BiVO4through GO, thereby achieving spatial isolation of the two carriers. As a result to some extent, direct recombination of electrons and holes was prevented.
Electrochemical impedance (EIS) is an effective method for analyzing the improved charge separation efficiency, which is also conducted in Fig. 6(c). It depicted the magnitude of the impedance arc diameter under sample illumination, g-C3N4>BiVO4>GO/g-C3N4> B2GC8, indicating that the B2GC8 ternary coupled photocatalyst has the ability to rapidly transfer and separate photogenerated carriers. This result is consistent with the effect of the RhB degradation experiment.
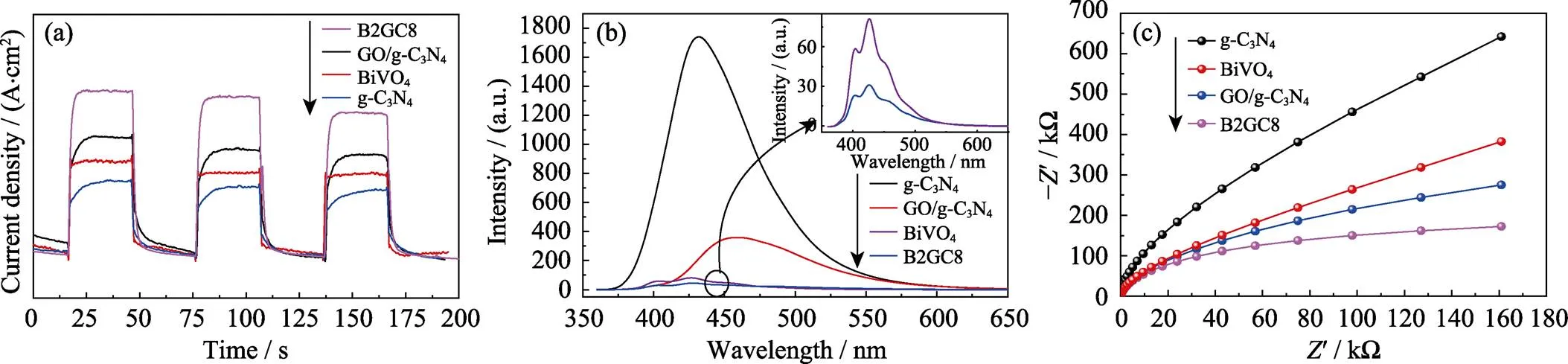
Fig. 6 Transient photocurrent responses (a), photoluminescence (PL) spectra (b), and EIS Nyquist plots (dot) (c) of g-C3N4, BiVO4, GO/g-C3N4 and BiVO4/GO/g-C3N4
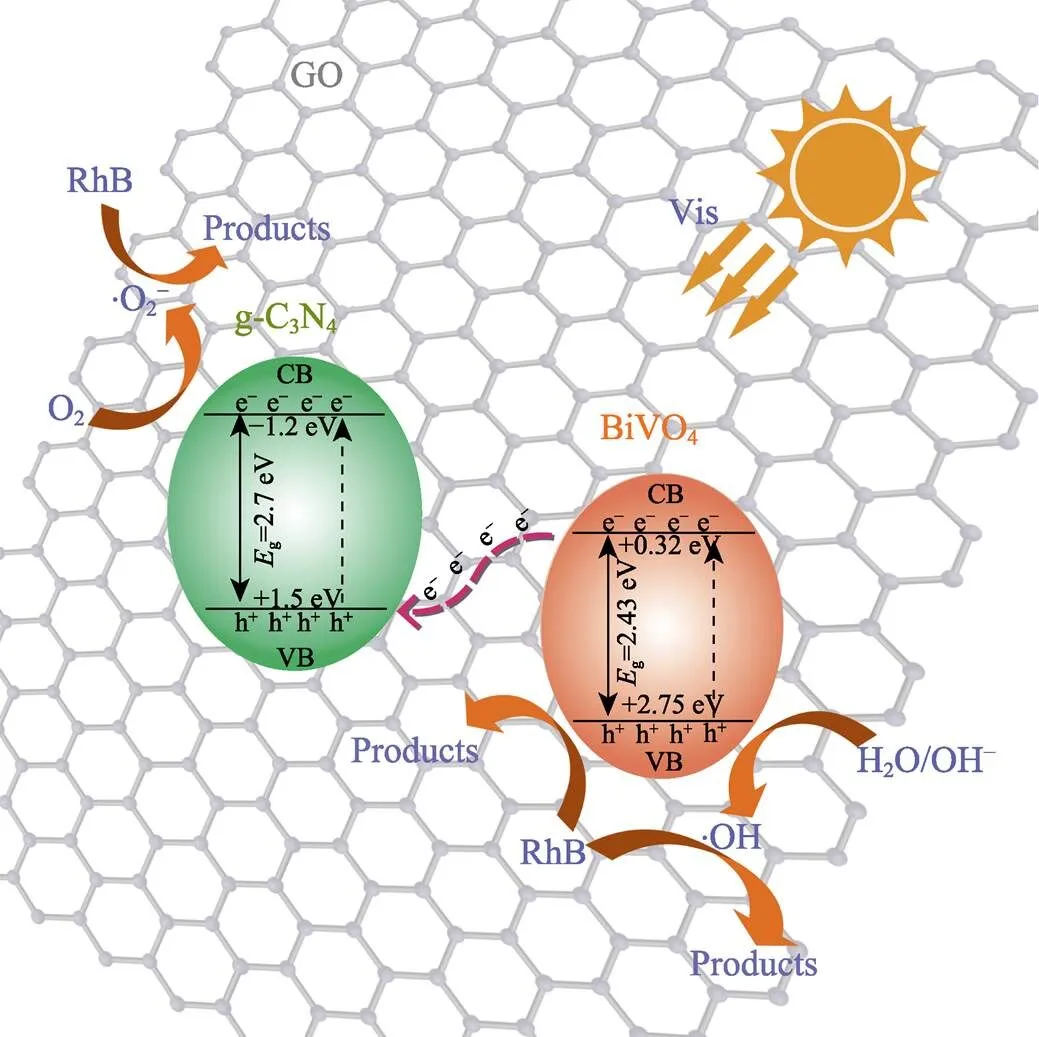
Fig. 7 Possible photocatalytic enhanced mechanism over BiVO4/GO/g-C3N4 Z-scheme photocatalysts under visible light irradiation
2.4 Photocatalytic mechanism analyses
Through the above characterization results and analysis, the potential photocatalytic mechanism of the Z-scheme BiVO4/GO/g-C3N4ternary photocatalyst was tentatively proposed, and the reaction process was illustrated in Fig. 7. Due to the energy band gap of 2.43 and 2.7 eV, BiVO4and g-C3N4can be excited even under visible light irradiation, resulting in electron hole separation. According to the different conduction band and valence band position of the BiVO4and g-C3N4, g-C3N4and BiVO4anchored on GO by strong interfacial electrostatic interaction to form a Z-scheme heterojunction. Under the interface Z-scheme heterostructure, the photogenerated electrons in the CB of BiVO4spontaneously move to the VB g-C3N4and BiVO4. The interface Z-scheme heterostructure results in spatial segregation of photogenerated carriers and then accumulates electrons and holes. The electrons in the CB of g-C3N4respectively generate ·OH radicals, and ·O2?has strong oxidation, and then induce RhB degradation together with the holes. Therefore, the photocatalytic performance of the g-C3N4/GO/BiVO4ternary heterostructure can be significantly enhanced.
3 Conclusions
A new Z-scheme photocatalyst of ternary BiVO4/GO/g- C3N4composites was successfully synthesized and employed for the photocatalytic degradation of RhB. In the photocatalytic process of BiVO4/GO/g-C3N4, GO nano-sheet act as fast transmission channels between BiVO4and g-C3N4and can suppress electron-hole recombination, which significantly promotes the charge separation and improves the redox ability of the ternary heterojunction. As a result, about 85% of RhB dye was degraded by the ternary composites after 120 min visible light irradiation, superior to that of the single or binary system. In addition, the enhanced degradation efficiency could be ascribed to the active h+species in degradation progress in trapping experiment. Overall, the Z-scheme BiVO4/GO/g-C3N4is an excellent photocatalyst with high-efficiency and has good potential for further application to organic-pollutants decomposition.
[1] Teh C M, Mohamed A R. Roles of titanium dioxide and ion- doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: a review., 2011, 509(5): 1648–1660.
[2] Ramya R, Krishnan P S, Krishnan M Neelaveni,Enhanced visible light activity of Pr-TiO2nanocatalyst in the degradation of dyes: effect of Pr doping and TiO2morphology., 2019, 19(7): 3971–3981.
[3] Riaz U, Ashraf S M, Kashyap J. Role of conducting polymers in enhancing TiO2-based photocatalytic dye degradation: a short review.,2015, 54(17): 1850–1870.
[4] Hunge Y M, Yadav A A, Mahadik M A,Degradation of organic dyes using spray deposited nanocrystalline stratified WO3/TiO2photoelectrodes under sunlight illumination., 2018, 76: 260–270.
[5] Pirhashemi M, Habibi-Yangjeh A, Rahim Pouran S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts., 2018, 62: 1–25.
[6] Massey A T, Gusain R, Kumari S,Hierarchical microspheres of MoS2nanosheets: efficient and regenerative adsorbent for removal of water-soluble dyes., 2016, 55(26): 7124–7131.
[7] He H, Zhou Y, Ke G,Improved surface charge transfer in MoO3/BiVO4heterojunction film for photoelectrochemical water oxidation., 2017, 257: 181–191.
[8] Zhao Z, Zhang W, Shen X,Preparation of g-C3N4/TiO2/ BiVO4composite and its application in photocatalytic degradation of pollutant from TATB production under visible light irradiation., 2018 358: 246–255.
[9] Wang K, Zhang G, Li J,0D/2D Z-scheme heterojunctions of bismuth tantalate quantum dots/ultrathin g-C3N4nanosheets for highly efficient visible light photocatalytic degradation of antibiotics., 2017,9(50): 43704–43715.
[10] Feng J, Gao M, Zhang Z,Comparing the photocatalytic properties of g-C3N4treated by thermal decomposition, solvothermal and protonation., 2018, 11: 331–334.
[11] Yan J, Song Z, Wang X,Enhanced photocatalytic activity of ternary Ag3PO4/GO/g-C3N4photocatalysts for Rhodamine B degradation under visible light radiation., 2019, 466: 70–77.
[12] Tan Y, Shu Z, Zhou J,One-step synthesis of nanostructured g-C3N4/TiO2composite for highly enhanced visible-light photocatalytic H2evolution., 2018, 230: 260–268.
[13] Xie Z, Feng Y, Wang F,Construction of carbon dots modified MoO3/g-C3N4Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline., 2018, 229: 96–104.
[14] Nie N, Zhang L, Fu J,Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2reduction performance., 2018, 441: 12–22.
[15] Li Y, Wu X, Ho W,Graphene-induced formation of visible- light-responsive SnO2-Zn2SnO4Z-scheme photocatalyst with surface vacancy for the enhanced photoreactivity towards NO and acetone oxidation., 2018, 336: 200–210.
[16] Deng Y c, Tang L, Zeng G m,Facile fabrication of mediator-free Z-scheme photocatalyst of phosphorous-doped ultrathin graphitic carbon nitride nanosheets and bismuth vanadate composites with enhanced tetracycline degradation under visible light.,2018, 509: 219–234.
[17] Wu Q, Bao S, Tian B,Double-diffusion-based synthesis of BiVO4mesoporous single crystals with enhanced photocatalytic activity for oxygen evolution., 2016, 52(47): 7478–7481.
[18] Wu X, Zhao J, Wang L,Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light., 2017, 206: 501–509.
[19] Liu Q, Guo Y, Chen Z,Constructing a novel ternary Fe(III)/graphene/g-C3N4composite photocatalyst with enhanced visible-light driven photocatalytic activityinterfacial charge transfer effect.,2016, 183: 231–241.
[20] Xiang Q, Yu J, Jaroniec M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4composites., 2011, 115(15): 7355–7363.
[21] Xue B, Jiang H Y, Sun T,ZnS@g-C3N4composite pho-to-catalysts:synthesis and enhanced visible-light photocatalytic activity., 2016, 146(10): 2185–2192.
[22] Huang Y, Zhang X, Zhu G,Synthesis of silver phosphate/sillenite bismuth ferrite/graphene oxide nanocomposite and its enhanced visible light photocatalytic mechanism., 2019, 215: 490–499.
[23] Zhang R, Huang Z, Li C,Monolithic g-C3N4/reduced graphene oxide aerogel with in situ embedding of Pd nanoparticles for hydrogenation of CO2to CH4., 2019, 475: 953–960.
[24] Dowla B M R U, Cho J Y, Jang W K,Synthesis of BiVO4-GO-PTFE nanocomposite photocatalysts for high efficient visible-light-induced photocatalytic performance for dyes., 2017, 28(20): 15106–15117.
[25] Lin H, Ye H, Chen S,One-pot hydrothermal synthesis of BiPO4/BiVO4with enhanced visible-light photocatalytic activities for methylene blue degradation., 2014, 4(21): 10968.
Z型BiVO4/GO/g-C3N4復合材料的制備及其可見光下催化性能
許世超1, 朱天哲1, 喬陽2, 白學健2, 唐楠1, 鄭春明2
(天津工業大學 1. 環境科學與工程學院; 2. 化學化工學院, 天津 300389)
Z-型光催化劑可以有效增強電荷分離, 從而改善光催化劑的活性。采用浸漬–煅燒和水熱法兩步制備Z型BiVO4/GO/g-C3N4光催化劑, 并用不同手段對其進行表征。在BiVO4/GO/g-C3N4的光催化過程中, GO納米片作為BiVO4和g-C3N4之間的快速傳輸通道, 可以抑制電子–空穴復合, 顯著促進電荷分離, 提高三元異質結的氧化還原能力。與單組分或二元復合物相比, 該催化劑具有良好的光降解羅丹明B(RhB)的能力。在可見光照射下, 它能夠在120 min內降解85% RhB, 空穴(h+)在反應中起主要作用。該工作為三元光催化劑體系提供了簡單的制備方法, 其中g-C3N4通過GO與BiVO4偶聯, 光催化活性顯著提高。
BiVO4; g-C3N4; GO; 三元催化劑; Z型異質結
TB34
A
date:2019-07-23;
date: 2019-11-05
National Natural Science Foundation of China (51772208, 51678409); Natural Science Foundation of Tianjin (17JCYBJC15900)
XU Shichao (1975–), male, PhD, associate professor. E-mail: xushichao@tjpu.edu.cn
許世超(1975–), 男, 博士, 副教授. E-mail: xushichao@tjpu.edu.cn
1000-324X(2020)07-0839-08
10.15541/jim20190380

