Thermal Behavior and Safety of Dihydroxylammonium 3,3′-dinitroamino-4,4′-azoxyfurazanate
XIAO Li-bai,GAO Hong-xu, ZHAO Feng-qi, REN Xiao-ning, QU Wen-gang, CHEN Xue-li, WANG Ying
(Science and Technology on Combustion and Explosion Laboratory, Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
Abstract:The thermal decomposition behaviors of dihydroxylammonium 3,3′-dinitroamino-4,4′-azoxyfurazanate(HNAF) were studied by using a C-500 type Calvet microcalorimeter at five different heating rates. The kinetic parameters of exothermic decomposition reaction were calculated by the Kissinger and Ozawa methods and then thermodynamic parameters were also determined. The specific heat capacity of dihydroxylammonium 3,3′-dinitroamino- 4,4′-azoxyfurazanate was measured with a Micro-DSCⅢ calorimeter.The results show that the apparent activation energy(E), pre-exponential constant(A), the entropy of activation(ΔS≠), the enthalpy of activation(ΔH≠) and the Gibbs free energy of activation(ΔG≠) are 205.26kJ/mol,1020.32s-1,140.76J/(mol·K), 201.56kJ/mol and 200.39kJ/mol, respectively. The specific heat capacity equation and the molar specific heat capacity were determined as Cp=-1.560+0.016T-2.263×10-5T2(J/(g·K)) and 446.028J/(mol·K), respectively. The self-accelerating decomposition temperature, adiabatic decomposition temperature rise, critical temperature of thermal explosion and adiabatic time-to-explosion are 444.44K, 2382.89K, 452.86K, 12.46s and 12.54s, respectively.
Keywords:physical chemistry; dihydroxylammonium 3,3′-dinitroamino-4,4′-azoxyfurazanate; thermal behavior; thermal safety
Introduction
With the modification and upgrading of the weapon and equipment, high-performance weapons make higher demands on energetic materials in modern war. So energetic materials with high energy, high density, good thermal stability, low moisture absorption, low toxicity, and low sensitivity to external stimuli have been increasingly paid attention by researchers all over the world[1-7]. So a variety of noval energetic materials have been constantly synthesized and researched[8-12].
Furazan compounds have very good performance of energy and sensitivity, therefore many kinds of furazan compounds were prepared and characterized[13-17]. Dihydroxylammonium 3,3′-dinitroamino-4,4′-azoxyfurazanate(HNAF) is a new highly energetic and insensitive furazan compound. It has high density (ρ=1.90g/cm3), good thermal stability (decomposition sets on above 175℃), high specific impulse(Isp=286s), low sensitivity to external stimuli (impact sensitivityH50=19J, friction sensitivity 120N), and desirable explosion parameters (detonation rateD=9511m/s, detonation pressurepcj=42.2GPa)[18]. In addition, its compatibility with some components of solid propellant has been explored[19]. In order to ensure the safety in process of practical application, thermal behavior and safety should be studied.
In this study, the thermal decomposition behaviors of HNAF were investigated by a C-500 type Calvet microcalorimeter at five heating rates. The kinetic and thermodynamic parameters were calculated. The self-accelerating decomposition temperature, the adiabatic decomposition temperature rise, the critical temperature of thermal explosion, and the adiabatic time-to-explosion were also obtained at the same time. The results can provide helpful information for its applications in explosive and propellant in the future.
1 Experiment
1.1 Sample
The sample(HNAF) with purity higher than 99.5% used in the thermal decomposition experiment was synthesized by Taiyuan University of Science and Technology and Xi′an Modern Chemistry Research Institute,and it was kept under vacuum before use.
1.2 Equipment and conditions
The specific heat capacity of HNAF was determined with a Micro-DSCⅢ calorimeter (SETARAM, France). The thermal decomposition curves were measured by means of a C-500 type Calvet microcalorimeter(SETARAM, France). The microcalorimeter was calibrated with the standard materials of In and Sn and the precision of enthalpy measurement was better than 0.5%.
2 Results and Discussion
2.1 Thermal decomposition behavior
The heat flow curves of HNAF at several heating rates are given in Fig. 1. It can be seen that the heat flow curve has only one exothermic peak and the peak temperature increases with increasing heating rate. The characteristic temperatures obtained by the heat flow curves at several heating rates are listed in Table 1.
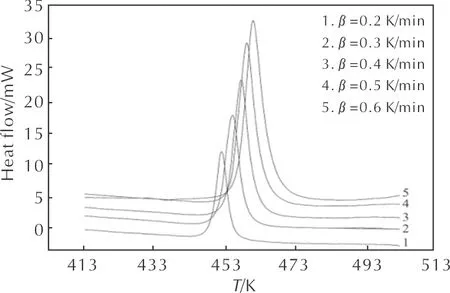
Fig.1 The heat flow curves of HNAF at different heating rates
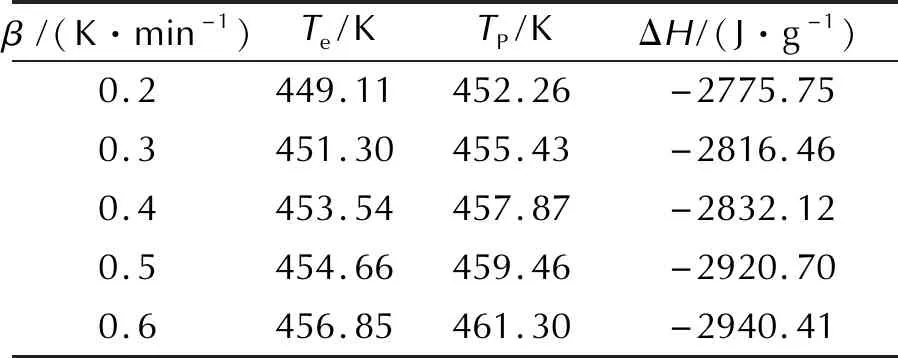
Table 1 Original data of the thermal decomposition reaction
Note:βis the heating rate;Teis the onset temperature;Tpis the peak temperature;ΔHis the ehthalpy of decomposition reaction.
The multiple heating methods (Kissinger method and Ozawa method)[20-22]are employed to calculate the kinetic parameters of the thermal decomposition. Kissinger equation and Ozawa equation are as follow[23-24]:
(1)
(2)
Whereβiis the linear heating rate,K/min;Tpirepresents the peak temperature of the thermal decomposition process,K;Ris the gas constant,J/(mol·K);Eis the apparent activation energy andAis the pre-exponential constant.
The values ofEandAcan be obtained by Kissinger method (with a subscript of k) and Ozawa method(with a subscript of o) according to the original data in Table 1. The apparent activation energy(Ek), pre-exponential constant(A) and correlation coefficient(rk) calculated by Kissinger method are 205.26kJ/mol, 1020.32s-1and 0.999, respectively. The apparent activation energy(Eo) and correlation coefficient(ro) calculated by Ozawa method are 202.42kJ/mol and 0.999. It can be seen that the apparent activation energy obtained by Kissinger method is in good agreement with that obtained by Ozawa method, and the linear correlation coefficients are all close to 1, which means that the results is reliable.
2.2 The specific heat capacity of HNAF
Fig. 2 shows the determination results of specific heat capacity of HNAF using continuous specific heat capacity mode of a Micro-DSCⅢ apparatus. The sample mass was 371.82mg and the heating rate was 0.2K/min from 10℃ to 60℃. In determined temperature range, specific heat capacity of HNAF presents a good linear relationship with temperature and the linear correlation coefficient is 0.993. The equation[25-26]describing the relationship can be written as:
Cp(J·g-1·K-1)=-1.560+0.016T-2.263×10-5T2 (3) The molar specific heat capacity is 446.028J/(mol·K)at 298.15K. Fig. 2 Specific heat capacity of HNAF The valueTe0andTp0ofTeandTpcorresponding toβ→0 can be obtained by substituting theTeiandTpifrom Table 1 into Eq.4 and the self-accelerating decomposition temperature (TSADT)[26-27]can be also obtained by Eq. 5: (4) TSADT=Te0 (5) Wherebandcare coefficients. The values ofTe0(TSADT) andTp0are 444.44K and 444.91K, respectively. With the help of the data ofEandAobtained by Kissinger method and Ozawa method, the entropy of activation(ΔS≠), the enthalpy of activation(ΔH≠), and the Gibbs free energy of activation(ΔG≠)[26-27]are obtained by eqs. 6-8 as 140.76J/(mol·K), 201.56kJ/mol, and 200.39kJ/mol corresponding toT=Tpo=444.91K,E=Ek=205260J/mol,A=Ak=1020.32s-1. The value of ΔG≠is positive, showing that the thermal decomposition reactions of HNAF will not take place until the proper temperature is reached. (6) ΔH≠=Ek-RT (7) ΔG≠=ΔH≠-TΔS≠ (8) WherekBis the Boltzmann constant andhis the Planck constant. The adiabatic decomposition temperature rise is defined as ΔTad. It can be obtained by Eq. 9[26]: (9) WhereHdis the quantity of heat released from the thermal decomposition, andCpis the specific heat capacity of HNAF. The specific heat capacity of HNAF wasCp=1.199J/(g·K) at 298.15K,Hdwas obtained from the average of ΔHat different heating rates, and the value is-2857.09J/g. The critical temperature of thermal explosion(Tb) is an important parameter of evaluating the safety and elucidating transition tendency from thermal decomposition to thermal explosion for small-scale EMS. For HNAF, the value ofTbobtained from Eq. 10[26-27]using the value ofTe0, and the valueTbis 452.86K. (10) It takes a certain time for energetic materials to begin thermal decomposition to thermal explosion in the adiabatic conditions. The time is named the adiabatic time-to-explosion.The adiabatic time-to-explosion is an important parameter for assessing their thermal stability and the safety. It can be calculated by the following Eqs.11-14[26-27]: (11) f(α)=(1-α)n (12) (13) Cp=a+bT+cT2 (14) Wheretis the adiabatic time-to-explosion,s;Cpis the specific heat capacity,J/(g·K);Ais the pre-exponential constant,s-1;Qis the exothermic value,J/g;Eis the apparent activation energy,J/mol;Ris the gas constant,J/(mol·K);αis the fraction of conversion;f(α) is the decomposition reaction mechanism function and the limit of temperature integral is fromTe0(K) toTb(K).The combination of Eqs.11-14 can give the following equation: For HNAF,Q=2857.09J/g,A=1020.32s-1,E=205260J/mol,Te0=444.44K,Tb=452.86K, Cp(J·g-1·K-1)=-1.560+0.016T-2.263×10-5T2, thent=12.46s(n=0),t=12.50s(n=1), andt=12.54s(n=2). We can see that the adiabatic time-to-explosion is little affected by the reaction order, so the adiabatic time-to-explosion of HNAF is a certain value between 12.46s and 12.54s. (1) The thermal decomposition behaviors of HNAF were studied by a C-500 type Calvet microcalorimeter at five heating rates and the values of enthalpy were calculated accurately. The apparent activation energy(E) and the pre-exponential constant(A) of the thermal decomposition for HNAF were determined by using Kissinger method and Ozawa method. (2) The standard molar specific heat capacity of HNAF is 446.028J/(mol·K) at 298.15K, and the equation describing values of the specific heat capacity versus temperatures is Cp(J·(g-1·K-1)=-1.560+0.016T-2.263×10-5T2(283.15K (3) The values of the thermodynamic parameters of HNAF of activation reaction ΔS≠, ΔH≠, ΔG≠are 140.76J/(mol·K), 201.56kJ /mol, and 200.39kJ/mol, respectively. (4) The self-accelerating decomposition temperature, the adiabatic decomposition temperature rise, and the critical temperature of thermal explosion of exothermic decomposition reaction are 444.44, 2382.89 and 452.86K, respectively. The adiabatic time-to-explosion of exothermic decomposition reaction is a certain value between 12.46s and 12.54s.
(283.15K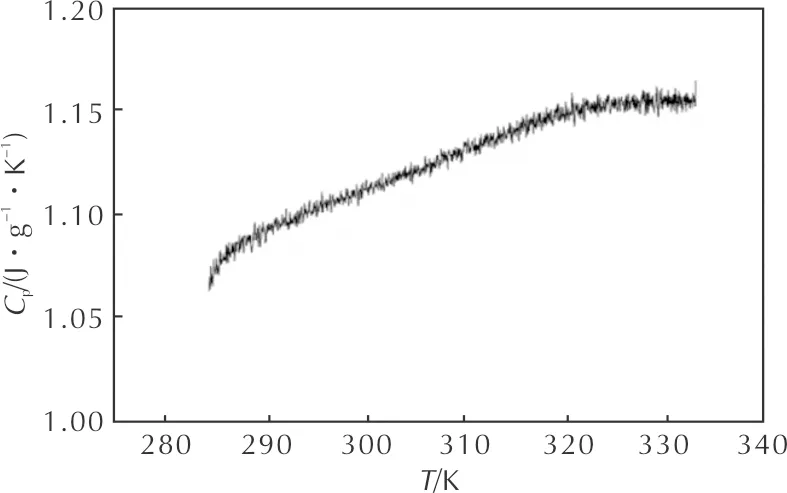
2.3 Self-accelerating decomposition temperature (TSADT)
2.4 Thermodynamic parameters of activation reaction
2.5 Adiabatic decomposition temperature rise(ΔTad)
2.6 Critical temperature of thermal explosion(Tb)
2.7 Adiabatic time-to-explosion
3 Conclusions

