The role of CALYPSO in the discovery of high-Tc hydrogen-rich superconductors?
Wenwen Cui(崔文文)and Yinwei Li(李印威)
Laboratory of Quantum Materials Design and Application,School of Physics and Electronic Engineering,Jiangsu Normal University,Xuzhou 221116,China
Keywords:CALYPSO,structure prediction,hydrogen-rich superconductors
1.Introduction
Superconductivity,one of the most intriguing material properties,has sparked countless studies since its discovery in 1911.[1]Superconductors are categorized as either conventional(if their behaviour can be explained by the Bardeen–Cooper–Schrieffer(BCS)theory[2]or its derivatives)or unconventional(otherwise). Based on BCS theory,materials with light elements are especially favourable for achieving superconductivity because these elements provide high frequencies in the phonon spectrum. This theory has underpinned the design of conventional high-Tcsuperconductors.For many years,the development of superconductors was restricted by the Mcmillan limit,[3]which states that the maximum Tcin conventional superconductors cannot exceed 40 K.To overcome this limit,scientists addressed the following two questions. Are there conventional superconductors with Tcabove 40 K in nature based on the known materials? What is the origin of the superconductivity? Ashcroft’s proposal that solid hydrogen(H2)and hydrogen-rich compounds represent candidates for high-Tcsuperconductors provided a turning point.[4,5]However,the metallization of solid hydrogen is challenging experimentally. A claim by Dias and Silvera to have observed atomic metallic hydrogen in the laboratory[6]remains controversial.[7,8]
Hydrogen-rich materials have become the main focus of superconductor research because metallization can be realized at lower pressures due to chemical precompression.[5]There are vast numbers of hydrides in nature, including many known hydrides and many new hydrides that can only be formed at high pressure. Therefore,discovering new hydrogen-containing superconductors by using traditional experimental methods based on trial and error is painstaking,and time-consuming work. Consequently,theoretical predictions are urgently required to guide the experimental synthesis of high-Tcsuperconductors. The key to designing H-containing superconductors at high pressure is to determine the crystal structures. Crystal structure AnaLYsis by Particle Swarm Optimization(CALYPSO)[9–11]is one of the most efficient structure prediction methods,and only needs the chemical composition and external conditions,such as pressure,to predict stable and metastable structures in combination with first-principles calculations.[12–18]CALYPSO has theoretically predicted large numbers of superconductors,[19,20]some of which have been confirmed experimentally.

Fig.1. Calculated and experimental Tc values of hydrogen-rich superconductors by year of publication.Solid red circles denote the calculated Tc of hydrogen-rich superconductors predicted by CALYPSO.Three experimental results are denoted by green squares,a blue triangle,and a pink diamond.
Given the accuracy and fruitful results of CALYPSO in searching for high-Tcsuperconductors,we present a review of the recent advances in the CALYPSO prediction of superconducting hydrides at high pressure(Fig.1).Experimentalists have attempted to obtain many predicted superconductors,among which hydrogen sulfides[21]and LaH10[22,23]have set record Tcvalues of 203 K and 250 K,respectively.
2.Superconductors predicted by CALYPSO
CALYPSO in combination with first-principles calculations has predicted many hydrides with high Tcvalues,including alkaline earth metal hydrides(e.g.,CaH6,[24]MgH6[25]),rare-earth metal hydrides(e.g.,YH6,[26]YH10,and LaH10[27,28]),transition metal hydrides(e.g.,NbH4,[29]VH8,[30]TcH2,[31]WH5,and WH6[32]),boron group hydrides (e.g., GaH3[33]), tetragen hydrides (e.g., SiH4[34]and PbH8[35]),pnictogen hydrides(e.g.,PH3,[36]P4H6,[37]and AsH8[38]),chalcogen hydrides(e.g.,H2S,[39]H4S3,[40]H3Se,[41]H4Te, and H5Te2[42]), halogen hydrides (e.g.,HBr[43]and HCl[44]),and noble gas hydrides(XeH2[45]).These hydrides have a wide range of Tcvalues up to 326 K.The compressed hydrogen sulfides[39]and lanthanum hydrides[27,28]have been synthesized experimentally.[21–23]Thus,we discuss these two breakthrough compounds in detail.

Fig.2.Typical structures predicted by CALYPSO in sulfur hydrides:(a) H2S(90 GPa),(b)Cmca H2S(170 GPa),(d)P212121 H3S4(25 GPa),and(d)Pnma H3S4(100 GPa). The small pink and large yellow spheres represent hydrogen and sulfur atoms,respectively.
2.1.Hydrogen sulphide:the first example of a predicted superconductor confirmed experimentally
As a typical molecular system,H2S crystallizes in three different phases at ambient pressure.[46,47]Under high pressure, more complicated phases emerge theoretically and experimentally,[48,49]but these remain elusive.[39]H2S was not initially identified as a promising superconductor because it was expected to dissociate into its constituent elements at high pressure(80 GPa)before metallization.[50,51]In 2014,CALYPSO predicted that H2S is thermodynamically stable up to at least 200 GPa.In addition,the structure predictions also identifeid two metallic phases with space groups(Fig.2(a))and Cmca(Fig.2(b))that are stable above 80 GPa and are superconductor candidates with estimated Tcaround 80 K.[39]Inspired by this prediction,Drozdov et al.[21]compressed the sulfur hydrides in a diamond anvil cell.They detected superconductivity by a sharp drop to zero resistance and magnetic susceptibility measurements.The measured Tcwas sensitive to temperature,which suggested that there was a low-Tcphase with Tcof 33–150 K at 110–220 GPa when the samples were prepared at low temperature(<100 K)and a high-Tcphase with Tcof 203 K when the sample was heated to room temperature.Drozdov et al.did not identify the two phases,however,they suggested that the low-Tcphase was H2S because it agreed well with our predicted results.[39]
Many subsequent studies investigated the origin of the Tcvalue of 203 K.Theoretical studies reported that the high-Tcphase originated from H3S,formed by the decomposition of compressed H2S at high pressure.[52–58]H3S was first synthesized in 2011 by Strobel et al.,and then was predicted as a superconductor with a high Tcof ~200 K,[50]close to the experimentally observed high Tcvalue.[21]The decomposition product of compressed H2S was studied under high pressure by first-principles structure predictions and x-ray diffraction(XRD)experiments.[40]In addition to H2S and H3S,CALYPSO predicted new stoichiometries of H2S3,H3S2,HS2,and H4S3.Based on these results,a possible dissociation path for H2S of 8H2S →S+4H3S+H4S3was proposed and confirmed by high-pressure XRD experiment.This was also the first demonstration of the partial decomposition of H2S into H3S at high pressure.The co-existence of H3S and H2S provided direct evidence that explained the experimental observation of two superconductive phases.The P212121and Pnma structures of H4S3are shown in Figs.2(c)and 2(d),respectively,and the Pnma phase is superconducting with Tcof ~2 K at 140 GPa.
The mechanism of the superconductivity at 203 K in compressed hydrogen sulfide at high pressure has been widely studied to aid the design of new high-Tcsuperconductors.[52,58–60]Several theoretical studies revealed that covalent bonding plays a key role in the large electron–phonon coupling.[61–63]To establish the relationship between Tcand covalent bond strength,we constructed a hypothetical compound,H6SSe,by substituting half of the S atoms in H3S with Se atoms.[62]Using the unbiased CALYPSO method,we identified three dynamically stable structures(Figs.3(b)–3(d)). These three structures retain the maincubic framework of H3S(Fig.3(a))with different Se substitution positions,leading to the formation of covalent S–H and Se–H bonds with different bond strengths.To investigate the effect of covalent bonding on the high Tcof chalcogen hydrides,we plotted Tcas a function of bond strength reflected by the Laplacian ?2ρ(Fig.3(e)).Tcdecreased from 195 to 115 K as the strength of the weakest covalent H–S or H–Se bond in each structure decreased,indicating that strong covalent bonds are important in determining the high Tcof the H3S system.
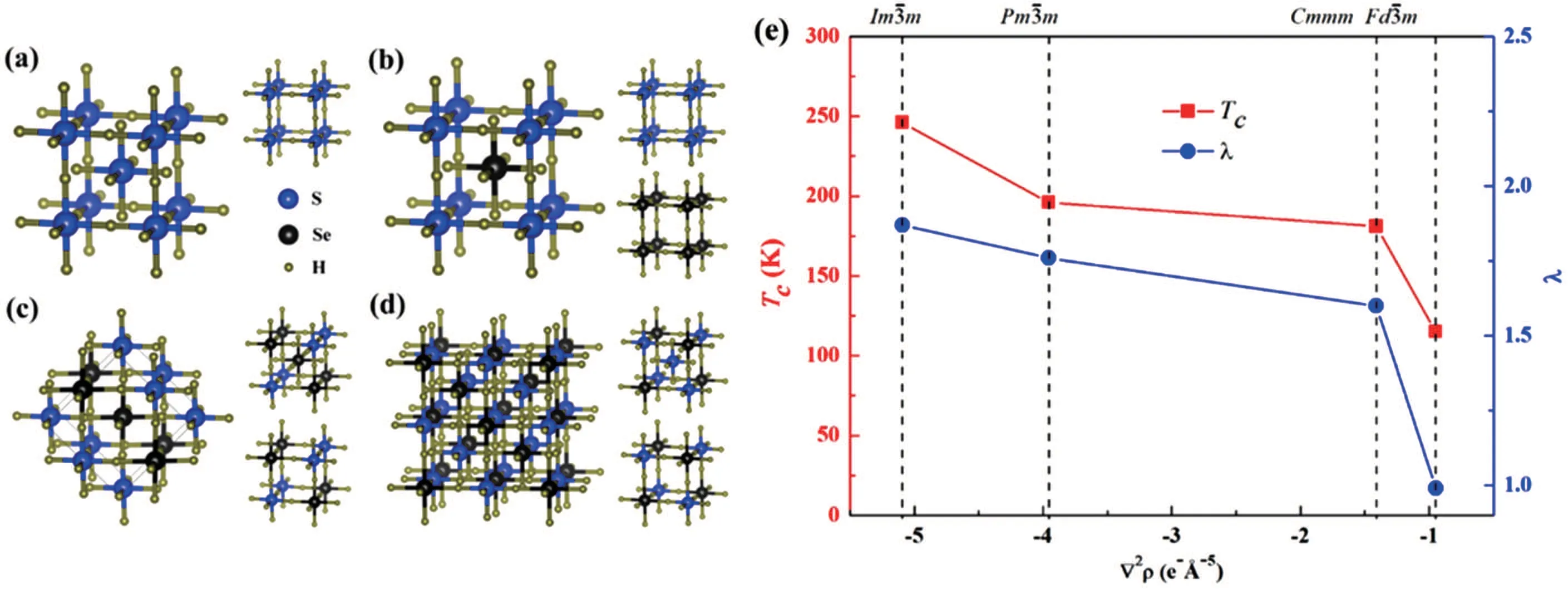
Fig.3.(a)structure of H3S and the predicted(b)(c)Cmmm,and(d) structures of H6SSe at 200 GPa.(e)Tχ and electron–phonon coupling constant λ as functions of bond strength of the weakest covalent bonds in H3S and H6SSe.
2.2.Clathrate hydrides:a leap to room-temperature superconductivity
2.2.1.LaH10
Hydrogen sulfide superconductivity is not the only prediction that has been confirmed experimentally.[21]In 2017,two separate CALYPSO studies of lanthanum hydride systems revealed new compounds with stoichiometries of LaH3,LaH4,LaH5,LaH8,and LaH10,as well as the known compound LaH2.[27,28]LaH10was predicted to be dynamically stable above 220 GPa with a face-centred cubic(fcc)structure(space groupthat was a unique H32clathrate-like structure,consisting of four H squares and 12 H hexagons(Fig.4(a)).The Tcof LaH10was estimated to be 257–274 K at 250 GPa,which is close to room temperature.The astonishing predicted Tcin LaH10prompted these hydrides to be experimentally synthesized. Just after the theoretical work was published,Geballe et al.[64]synthesized the lanthanum superhydrides LaH10±x(?2 ≤x ≤1)by directly compressing La and H2samples at 170 GPa and 1000 K.They subsequently published a different synthesis route using ammonia borane(NH3BH3)as the hydrogen source,[22]which produced LaH10±xat pressures of 180–200 GPa.They observed sharp drops in resistivity when they cooled the samples to 260 K,which indicates the superconducting transition. Drozdov et al.[23]also reported the superconductivity of fcc LaH10with Tcof 250 K at 170 GPa,synthesized by direct reaction of lanthanum and hydrogen under high pressures. The superconductivity was supported by the observation of zero resistance,the isotope effect,and a decrease in Tcupon the application of an external magnetic field.Previously,they had reported a Tcof 215 K in lanthanum hydrides,[65]which may correspond to other LaHxphases.The measured Tcof 250–260 K from the two independent experiments agree well with the predicted Tcof LaH10,[27,28]which demonstrates that CALYPSO is a powerful tool in the search for high-Tcsuperconductors.

Fig. 4. Clathrate hydrides predicted by CALYPSO. (a) La(y)H10,(b)Im3m Ca(y)H6,(c)P63/mmc Sc(Y,Ce)H9,and(d)Li2MgH16.[66]The building block metal atoms centred in the H32,H24,H29,H18 and H28 cages are also shown in each panel.
2.2.2.Other clathrate hydrides
LaH10was not the first structure containing an H-cage to be predicted. In 2012,Ma’s group used CALYPSO to predict that a new calcium hydride,CaH6,could be synthesized by compressing elemental calcium and hydrogen or CaH2and hydrogen.[24]In the CaH6structure,hydrogen atoms linked by weak covalent bonds form a clathrate with a calcium atom at the centre(Fig.4(b)).This unique body-centred cubic structure had a predicted Tcof 235 K at 150 GPa,which was the first time that a Tcof more than 200 K was predicted for a hydride.Later,this type of structure was also found in yttrium hydrides.In 2015,using CALYPSO,we predicted that a yttrium atom can react with six hydrogen atoms to form YH6at 120 GPa with the same structure as CaH6and a higher Tcof 264 K,[26]approaching room temperature. In 2017,in the same study that predicted LaH10,YH10was predicted to have a Tcof up to 305–326 K at 250 GPa,surpassing room temperature.[27,28]Ref.[28]also found that H atoms could form different cages in the rare earth(RE=Sc,Y,La,Ce,Pr,etc.)hydrides,such as,H24in REH6,H29in REH9,and H32in REH10(Fig.4).Surprisingly,it is also demonstrated that the calculated Tcfor the clathrates increases with increasing H cage size.For example,YH6,YH9,and YH10have Tcof 264 K(120 GPa),276 K(150 GPa),and 303 K(400 GPa),respectively.Other RE hydrides,LaH9,CeH9,CeH10,and PrH9,had much lower Tc(<56 K)due to the higher mass of the RE elements,although ScH6and ScH9had Tcof ~190 K.Liang et al.used CALYPSO to predict the ternary clathrate hydride,CaYH12,[67]which had a similar H clathrate structure to YH6and CaH6and possessed a Tcof 258 K at 200 GPa. More recently,Ma’s group predicted a clathrate structure in ternary hydride,Li2MgH16[66]with space group(Fig.4(d)),which contains Li-centered H18cages and Mg-centered H28cages. In particular,it exhibits a Tcof around 473 K at 250 GPa,the highest Tcin all the hydrides,which provides the possibility to obtain superconductivity even higher than room temperature.
3.Other superconductors
Owing to the high-Tcsuperconductivity of hydrogen sulfide,hydrides of elements neighbouring S,such as P,[36,38,68,69]Se,[41]and Te,[42]have been investigated.
3.1.Phosphorus hydrides
Just after the experimental observation of superconductivity in compressed H2S,Drozdov et al.compressed PH3,[70]an analogue of H2S,and observed a high Tcof ~100 K around 200 GPa,indicated by the abrupt drop to zero resistance.However,they did not identify the origin of the superconducting phase.Analogous to the decomposition of hydrogen sulfides,PH3may also decompose to new P–H compounds. Hence,it is important to search for new structures and stoichiometries to explain the origin of the Tcof ~100 K.CALYPSO structure prediction has been vital in exploring the origin of the superconductivity in phosphorous hydrides.In 2016,Liu et al.performed a structure search in the PHx(x=1,2,3,4,5)system at high pressure with CALYPSO.[36]The P212121(<210 GPa,Fig.5(a))andC2/m(>210 GPa,Fig.5(b))phases of PH3were discovered at high pressure. The calculated Tcof the C2/m phase was 83 K,which agreed well with the experimental results.However,these two phases were predicted to be energetically unstable,and decomposed into P and H at high pressure.
Wang’s group explored the decomposition of PH3at high pressure via a combination of experiments and CALYPSO prediction.[37]XRD and Raman measurements showed that PH3underwent decomposition at high pressures to produce a new stable compound,P4H6. P4H6is generated stepwise.First,dimerized PH3decomposes to P2H4:2PH3→P2H4+H2. Then,P4H6is generated by the further decomposition of P2H4:2P2H4→P4H4+H2. At low temperatures,P4H6can be observed up to 200 GPa.[70]However,the exact structure could not be determined experimentally.CALYPSO predicted the metallic structures of P4H6to be Cmcm(<182 GPa,Fig.5(c))and C2/m(>182 GPa,Fig.5(d)).In addition,the Tcof the C2/m phase was estimated to be 67 K at 200 GPa,which agreed with the measured Tc,indicating that P4H6could be the superconductor observed experimentally by Drozdov et al.[70]
3.2.Other chalcogen hydrides
In 2016,Ma’s group used CALYPSO to predict three metallic stoichiometries of HSe2,HSe,and H3Se,all of which exhibit superconductivity.[41]H3Se had the same cubic structure(Fig.3(a))as H3S and had a predicted Tcof 110 K at 250 GPa.Although H3Se and H3S have the same structures,the spectral functional are different because the Se atoms are heavier,which contributes to the lower Tc.In the same year,they identified three metallic stoichiometries of H4Te,H5Te2,and HTe3.Unlike the covalent bonds of H–S(Se)in H3S(Se),the H–Te bonds in tellurium hydrides are ionic bonds.[42]Especially,P6/mmm H4Te(Fig.6(a))contains elongated“H2”molecules,which is a superconductor with estimated Tcof 104 K at 170 GPa. While H5Te2,with space group C2/m(Fig.6(b)),has a relatively lower Tcof 58 K at 200 GPa.
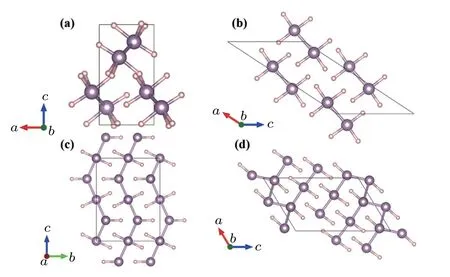
Fig.5.Structures of phosphorus hydride compounds predicted by CALYPSO.(a)P212121 PH3,(b)C2/m PH3,(c)Cmcm P4H6,and(d)C2/m P4H6.
3.3.Other hydrides
In addition to the typical hydride superconductors,CALYPSO has predicted other hydrogen-containing superconductors (Fig. 1) such as GaH3,[33]NbH4,[29]BeH2,[71]W–H,[32]and MgSiH6.[75]However,their Tcvalues are low(10–140 K).Thus,we do not discuss them in detail in this review.
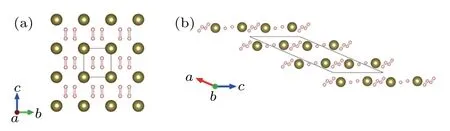
Fig.6. Structures of tellurium hydrides predicted by CALYPSO.(a)P6/mmm H4Te and(b)C2/m H5Te2.
4.Summary and outlook
We have summarized progress in high-Tcsuperconductors predicted by CALYPSO,and described the prediction of H2S and LaH10,which stimulated subsequent experimental studies. The observed Tcvalues of these compounds of 250–260 K pave the way to room-temperature superconductivity.Crystal structure prediction has been important in finding high-Tcsuperconductors. Because theorists have calculated Tcof binary hydrides for most elements in the periodic table,research can now focus on ternary hydrides as the next area in which to discover superconductors,however,the computational burden for these studies will be far higher.Experimentalists still face the challenge of synthesizing theoretically predicted materials.Furthermore,reducing the pressures at which superconducting phases appear is also a major challenge,so that superconductivity can be observed outside of diamond anvil cells and used in practical applications.The search for superconductors with higher Tcis another area of research.For example,predicting clathrate structures with larger cages may be a possible route to higher-Tcsuperconductors.
- Chinese Physics B的其它文章
- Theoretical analyses of stock correlations affected by subprime crisis and total assets:Network properties and corresponding physical mechanisms?
- Influence of matrigel on the shape and dynamics of cancer cells
- Benefit community promotes evolution of cooperation in prisoners’dilemma game?
- Theory and method of dual-energy x-ray grating phase-contrast imaging?
- Quantitative heterogeneity and subgroup classification based on motility of breast cancer cells?
- Designing of spin filter devices based on zigzag zinc oxide nanoribbon modified by edge defect?

