2-(5-甲基-1,3,4-噻二唑)-硫乙酸鋅配合物的合成、晶體結構和性質
潘兆瑞鄭和根
2-(5-甲基-1,3,4-噻二唑)-硫乙酸鋅配合物的合成、晶體結構和性質
潘兆瑞*,1,2鄭和根*,1
(1南京大學化學化工學院,配位化學國家重點實驗室,南京210023)
(2南京曉莊學院環境科學學院,南京211171)
合成了2個鋅配合物[Zn(mtyaa)2(H2O)4]·4H2O(1)和[Zn(bpe)(mtyaa)2(H2O)2]n(2)(Hmtyaa=2-(5-甲基-1,3,4-噻二唑)-硫乙酸;bpe=1,2-雙(4-吡啶基)乙烷),用X射線單晶衍射儀測定了配合物的單晶結構,并對它進行了元素分析、紅外光譜、熱重和粉末X射線衍射等表征。配合物1和2的晶體分別屬于三斜晶系和單斜晶系,空間群分別為P1和C2/c。π-π相互作用以及配位水和游離水分子與羧基氧之間的氫鍵作用將配合物1的單分子結構連成三維網狀結構。配合物2中配位水與羧基氧以及配體中的氮原子之間的氫鍵作用將相鄰鏈連接成二維平面結構。
2-(5-甲基-1,3,4-噻二唑)-硫乙酸;晶體結構;π-π相互作用;氫鍵
0 Introduction
The construction of supramolecular architectures are currently of great interest owing to their intriguing structures[1-4]and potential applications in catalysts, electrochemistry,optics,magnetism,gas sorption and so on[5-9].However,how to construct appropriate crystal structuresistheprimaryissue.Thecarboxylateligandsarewidelyemployedinthedesignof coordination frameworks for the following reasons: Firstly,because their various coordination modes and flexible molecular backbones can provide a variety of coordination polymers with appealing structures and properties[10].Secondly,the inherent negative charge of the carboxylate groups can compensate for the charge induced by the metal centers[11-12].Thirdly,hydrogen bond formation by carboxylate groups reinforces whole architectures[13-15].On the other hand,the N-donor ligands,such as 4,4′-bipyridine(4,4′-bipy)and 1,2-bis(4-pridyl)ethane(bpe)have also been proven to be usefulconnectorstoformulatehighdimensional compounds[16].Recently,we have successfully synthesized some supramolecular compounds by using 2-(5-methyl-1,3,4-thiadiazol-2-ylthio)acetic acid(Hmtyaa) ligand[17-19].Herein,we successfully obtained two new coordination compound based on Hmtyaa ligand and N-donor ligands(bpe),namely,[Zn(mtyaa)2(H2O)4]· 4H2O(1)and[Zn(bpe)(mtyaa)2(H2O)2]n(2).
1 Experimental
1.1 Reagents and physical measurements
Hmtyaaligandwassynthesizedasliterature reported[20].All other chemicals were of reagent grade qualityfromcommercialsourcesandwereused without further purification.The IR absorption spectra of the compounds were recorded in the range of 400~4 000 cm-1by means of a Nicolet(Impact 410)spectrometer with KBr pellets(5 mg of sample in 500 mg of KBr).C,H and N analyses were carried out with a Perkin Elmer 240C elemental analyser.XRD measurements were performed on a Philips X′pert MPD Pro X-ray diffractometer using Cu Kα radiation(λ=0.154 18 nm,2θ=5°~50°),in which the X-ray tube was operated at 40 kV and 40 mA.The as-synthesized samples were characterized by thermogravimetric analysis(TGA)on a Perkin Elmer thermogravimetric analyser Pyris 1 TGA up to 1 023 K using a heating rate of 20 K·min-1under a N2atmosphere.
1.2 Synthesis
1.2.1 Synthesis of[Zn(mtyaa)2(H2O)4]·4H2O(1)
A solution of ZnCl2(54 mg,0.4 mmol)in H2O(3 mL)was added to a solution of Hmtyaa(38 mg,0.2 mmol)in H2O(5 mL)which was adjusted to pH≈7.0 with dilute sodium hydroxide(1 mol·L-1),and the mixture was stirred at room temperature for 12 h to give a colorless solution,which was then filtered.Slow evaporation of the filtrate gave white block-shaped single crystals in two weeks.Yield:39.98 mg(68%). Anal.Calcd.for C10H26N4O12S4Zn(%):C,20.43;H, 4.46;N,9.53.Found(%):C,20.45;H,4.47;N,9.51.IR (KBr,cm-1):3 487(s),3 342(s),1 589(vs),1 401(m), 1 388(vs),1 248(m),1 206(w),1 093(w),904(w),781 (w),689(m),611(m),405(w).
1.2.2 Synthesis of[Zn(bpe)(mtyaa)2(H2O)2]n(2)
A solution of ZnCl2(27 mg,0.2 mmol)and bpe (36 mg,0.2 mmol)was added to a solution of Hmtyaa (38 mg,0.2 mmol)in H2O(7 mL)which was adjusted to pH≈7.0 with dilute sodium hydroxide(1 mol·L-1). The final mixture was sealed in a 15 mL PTFE-lined stainless-steel acid digestion bomb and heated at 75℃for 15 h.Little red crystals were obtained and filtered.The filtrate was allowed to slowly evaporate at ambient temperature over 3 days,and quantities of white triangular prism crystals were collected in 95% yield.Anal.Calcd.for C22H26N6O6S4Zn(%):C,39.79; H,3.95;N,12.65.Found(%):C,39.81;H,3.96;N, 12.62.IR(KBr,cm-1):3 427(s),1 615(vs),1 590(vs), 1 428(m),1 384(vs),1 219(w),1 198(w),1 074(m), 826(w),685(w),621(w),545(w).
1.3 X-ray crystallography
X-ray crystallographic data of both compounds were collected at room temperature using epoxy-coated crystals mounted on glass fiber.All measurements were made on a Smart CCD diffractometer with graphitemonochromated Mo Kα radiation(λ=0.071 073 nm)by using φ-ω scan mode at room temperature.The structures were solved by direct methods and refined with the full-matrix least squares technique using the SHELXS-97 and SHELXL-97 programs[21-22].Anisotropic thermal parameters were assigned to all non-hydrogen atoms.The hydrogen atoms bonding to C and N atoms were fixed geometrically at calculated distances and allowed to ride on the parent atoms,and the hydrogen atoms of water molecules were either found from thedifference Fourier map or fixed stereochemically. Crystallographicdataandstructuralrefinement parameters are summarized in Table 1,and the selected bond lengths and angles are given in Table 2 and 3.
CCDC:653462,1;653467,2.

Table1 Crystallographic data and structure refinement details for compounds 1 and 2

Table2 Selected bond lengths(nm)and angles(°)in compounds 1 and 2
Symmetry codes:a:-x+1,-y+2,-z+2 for 1;a:-x+1/2,-y+3/2,-z+1 for 2.

Table3 Hydrogen-bonding parameters in compounds 1 and 2
2 Results and discussion
2.1 Structure description of compounds 1 and 2
2.1.1 Crystal structure of[Zn(mtyaa)2(H2O)4]·4H2O(1) Each Znion is six coordinated and exhibits a distorted octahedral geometry.Two oxygens O2 and O2a from two ligands and two coordinated water donor O4 and O4a form the equatorial plane with an average length of 0.205 17 nm and an average O2-Zn1-O4 angle of 90.00(1)°,and the other two water molecules O3,O3a,occupy axial positions with an average length of 0.217 0(2)nm and the O3-Zn1-O3a angle of 180°(Fig.1).As depicted in Fig.1,mtyaa ligand acts as a monodentate ligand.Numerous hydrogen-bonding interactions occurred such as O(water)…O(carboxyl), O(water)…O(water),O(water)…N and O(water)…S, connect compound 1 into a 2D structure network(Fig. 2).The center-to-center separation for the parallelarranged ring are 0.354 and 0.344 nm,respectively, indicating the presence of strong face-to face π-π stacking interactions in compound 1,which further stabilize the 2D crystal structure.The two dimensional planar is further connected into three dimensional network by hydrogen bonds(Fig.3).

Fig.1 Structural unit of[Zn(mtyaa)2(H2O)4]·4H2O(1)with atom numberings

Fig.2 Two dimensional planar structure of compound 1 formulated by hydrogen bonds and π-π stacking interactions
2.1.2 Crystal Structure of[Zn(bpe)(mtyaa)2(H2O)2]n(2)
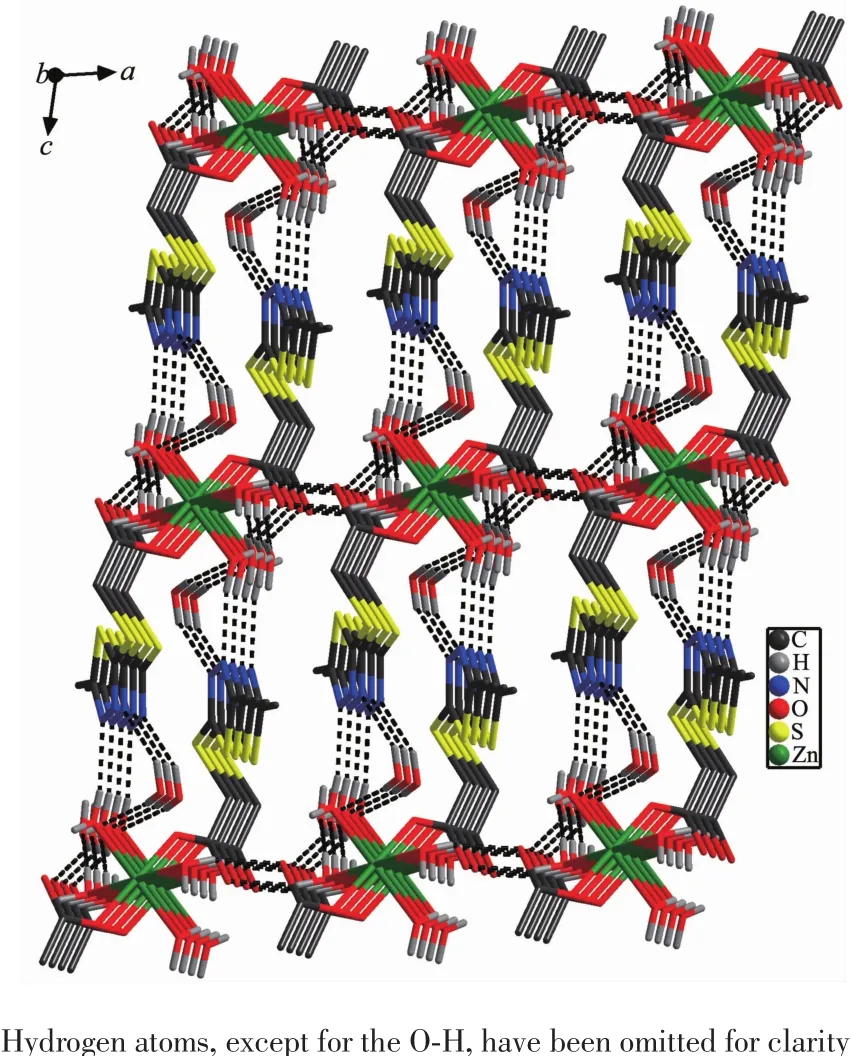
Fig.3 Three dimensional network formed through the O(water)…O(carboxyl)and O(water)…N hydrogen bonds in 1 viewed along b axis

Fig.4 Structural unit of{[Zn(bpe)(mtyaa)2(H2O)2]}n(2) with atom numberings
As illustrated in Fig.4,in compound 2,each Znis six coordinated and exhibits a distorted octahedral geometry.Two carboxylate oxygens O2 and O2a from twomtyaaligandsandtwocoordinatedwater molecules O3 and O3a form the equatorial plane with an average length of 0.213 38(18)nm and the average angle ofO(2)-Zn1-O(3)is 90.00°.Two nitrogen molecules N3 and N3a from two bpe ligands occupy axial positions with the length of 0.216 2(2)nm and the N3-Zn1-N3a angle of 180°.The adjacent Znions are bridged into one dimensional chain structure by nitrogen atoms coming from mtyaa ligands(Fig.5). Numerous hydrogen-bonding interactions such as O (water)…O(carboxyl)and O(water)…N,connect the adjacent chains into three dimensional network(Fig.6 and 7).
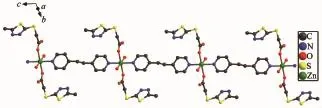
Fig.5 One dimensional chain structure of compound 2

Fig.6 Intra and inter chain hydrogen bonds of compound 2
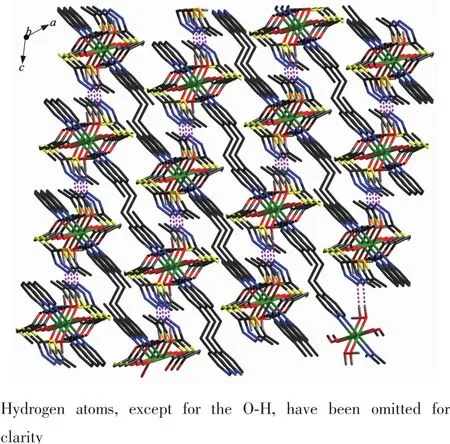
Fig.7 Three dimensional network formed through the O(water)…O(carboxyl)and O(water)…N hydrogen bonds in 2 viewed along b axis
2.2 Thermal and XRD analysis
To confirm whether the crystal structures are trulyrepresentativeofthebulkmaterials,XRD experiments were carried out for 1 and 2.The XRD experimental and computer-simulated patterns of the corresponding compounds are shown in Fig.8,which show that the bulk synthesized materials and the measured single crystals are the same.In order to investigate the thermal stability of the complex,their thermal behaviours were studied by TGA(Fig.9).For compound 1,a rapid weight loss is observed from 30to 159℃,which is attributed to loss of the coordinated water molecules and free water,with a weight loss of 24.28%(Calcd.24.49%).The TGA curve of 2 shows that 2 undergoes dehydration between 78 and 135℃, which is attributed to loss of the lattice water molecules, with aweight loss of 5.28%(Calcd.5.42%).The decomposition of the anhydrous residue of 1 and 2 occurs at 221 and 198℃,respectively.
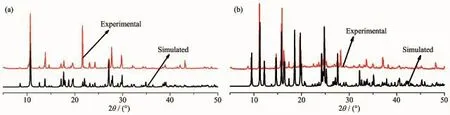
Fig.8 Experimental and simulated powder X-ray diffraction patterns of compound 1(a)and compound 2(b)at 293 K

Fig.9 TGA curves of compounds 1(a)and 2(b)
3 Conclusions
In summary,by using N-donor ligand(bpe)and Hmtyaa ligand,we have synthesized two new coordination compounds[Zn(mtyaa)2(H2O)4]·4H2O(1)and [Zn(bpe)(mtyaa)2(H2O)2]n(2).Bothcompoundsare connected into three dimensional network by hydrogen bonds between coordinated and free water molecules with carboxylate oxygen atoms.
[1]Qian J J,Jiang F L,Yuan D Q,et al.Chem.Commun.,2012, 48:9696-9698
[2]Van de Voorde B,Bueken B,Denayer J,et al.Chem.Soc. Rev.,2014,43:5766-5788
[3]Lei C,Chao S T,Wu H K,et al.Z.Kristallogr.-New Cryst. Struct.,2016,231:405-406
[4]Yu J C,Cui Y J,Wu C D,et al.J.Am.Chem.Soc.,2015, 137:4026-4029
[5]Xu Z,Han L,Zhuang L,et al.Inorg.Chem.,2015,54:4737-4743
[6]Banerjee D,Hu Z C,Li J.Dalton Trans.,2014,43:10668-10685
[7]Wang M S,Guo S P,Li Y,et al.J.Am.Chem.Soc.,2009, 131:13572-13573
[8]Jariwala D,Sangwan V K,Lauhon L J,et al.Chem.Soc. Rev.,2013,42:2824-2860
[9]Getman R B,Bae Y S,Wilmer C E,et al.Chem.Rev.,2012, 112:703-723
[10]Chu Q,Su Z,Fan J,et al.Cryst.Growth Des.,2011,11: 3885-3894
[11]Liu Y H,Lu Y L,Wu H C,et al.Inorg.Chem.,2002,41: 2592-2597
[12]Wei Y L,Hou H W,Li L K,et al.Cryst.Growth Des.,2005, 5:1405-1413
[13]Desiraju G R.Crystal Engineering:the Design of Organic Solids.Amsterdam:Elsevier,1989.
[14]Desiraju G R,Steiner T.Weak Hydrogen Bond in Structural Chemistry and Biology.Oxford:Oxford University Press, 1999.
[15]Desiraju G R.Angew.Chem.Int.Ed.,1995,34:2311-2327
[16]Ahmad M,Sharma M K,Das R,et al.Cryst.Growth Des., 2012,12:15711578
[17]Ma M H,Pan Z R,Xu J,et al.Chin.J.Struct.Chem.,2010, 29(6):843-852
[18]PAN Zhao-Rui(潘兆瑞),ZHOU Hong(周宏),XIE Hua(鮮華).Chinese J.Inorg.Chem.(無機化學學報),2010,26(11): 1955-1960
[19]PAN Zhao-Rui(潘兆瑞).Chinese J.Inorg.Chem.(無機化學學報),2010,27(10):2027-2032
[20]Srogl J,Liu W S,Marshall D,et al.J.Am.Chem.Soc., 1999,121:9449-9450
[21]Sheldrick G M.Acta Crystallogr.Sect.A,1990,A46:457
[22]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of G?ttingen,Germany,1997.
Syntheses,Crystal Structures and Properties of 2-(5-Methyl-1,3,4-thiadiazol-2-ylthio ZincCoordination Compounds
PAN Zhao-Rui*,1,2ZHENG He-Gen*,1
(1State Key Laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210023,China)
(2School of Environmental Science,Nanjing Xiaozhuang University,Nanjing 211171,China)
Two coordination compounds[Zn(mtyaa)2(H2O)4]·4H2O(1)and[Zn(bpe)(mtyaa)2(H2O)2]n(2)(Hmtyaa=2-(5-methyl-1,3,4-thiadiazol-2-ylthio)acetic acid,bpe=1,2-bis(4-pridyl)ethane)have been synthesized and they were structurally characterized by single-crystal X-ray diffraction,and then were characterized by elemental analysis, FTIR spectra,thermal analysis and powder X-ray diffraction.Compounds 1 and 2 crystallize in the triclinic and monoclinic,space group P1 and C2/c,respectively.Face-to-face π-π interactions and hydrogen bonds between coordinated and free water molecules with carboxylate oxygen atoms connect single molecules in compound 1 into three dimensional network.Compound 2 is connected into three dimensional structure by hydrogen bonds between coordinated water molecules and carboxylate oxygen atoms or nitrogen atoms.CCDC:653462,1;653467,2.
2-(5-methyl-1,3,4-thiadiazol-2-ylthio)acetic acid;crystal structure;π-π interaction;hydrogen bonds
O614.24+1
A
1001-4861(2017)09-1678-07
10.11862/CJIC.2017.207
2017-05-11。收修改稿日期:2017-06-19。
國家青年科學基金(No.21301094)資助項目。
*通信聯系人。E-mail:pzr_2006@163.com,zhenghg@nju.edu.cn;會員登記號:S06N1394M1405。

