Bi2WO6/TiO2納米管異質結構復合材料的多模式下的光催化活性比較
馬鳳延楊陽李娜楊麒麟李尚錦申路嚴
Bi2WO6/TiO2納米管異質結構復合材料的多模式下的光催化活性比較
馬鳳延*,1楊陽1李娜1楊麒麟1李尚錦2申路嚴2
(1齊齊哈爾大學化學與化學工程學院,齊齊哈爾161006)
(2齊齊哈爾大學材料科學與工程學院,齊齊哈爾161006)
以TiO2納米管為模板,采用多組分自組裝結合水熱法制備Bi2WO6/TiO2納米管異質結構復合材料。通過多種技術如X射線衍射(XRD),X射線光電子能譜(XPS),N2吸附-脫附,掃描電鏡(SEM),高分辨透射電鏡(HRTEM)和紫外可見漫反射吸收光譜(UV-Vis DRS)考察所制備樣品的組成、結構、形貌、光吸收和電子性質。Bi2WO6納米片或納米粒子分布在TiO2納米管上,形成異質結構。隨后,通過在紫外、可見和微波輔助光催化模式下降解染料羅丹明B(RhB)來評價復合催化劑的光催化活性。與TiO2納米管和Bi2WO6相比,Bi2WO6/TiO2-35納米管在多模式下表現出更優異的光催化活性。與紫外和可見降解模式相比,Bi2WO6/TiO2-35納米管在微波輔助光催化模式下對RhB的降解效率最高。這種增強的光催化活性源于適量Bi2WO6的引入、納米管獨特的形貌特征和降解模式所引起的增強的量子效率。降解過程中的活性物種被證明是h+,·OH和·O2-自由基。而且,在微波輔助光催化模式下,可產生更多的·OH和·O2-自由基。
TiO2納米管;Bi2WO6;多模式降解;光催化
0 Introduction
Titanium dioxide(TiO2)has attracted a great deal of research attention because of their potential applications in the photodegradation of organic pollutants, photocatalytic water splitting for hydrogen generation, dye-sensitized solar cells,and even gas sensors and biosensors,due to its low cost and abundant elements (Ti and O),long-term stability,and environmentalfriendly characteristics[1-3].However,its wide band gap and fast recombination of the photogenerated electronhole(e--h+)are two limitations for its contemporary applications[4].
To overcome the above limitations,some measures have been taken.At present,one effective approach is to adjust TiO2morphology.One-dimensional(1D)TiO2nanomaterialshavebeenreceivingextensiveinterests[5-8]. Moreover,compared with other forms of TiO2,titania nanotubespossessthedistinguishingfeaturesof nanotubes including large specific surface area,good electron/proton conductivity,and high aspect ratio.In addition,the open mesoporous morphology of TiO2nanotubes can efficiently transfer the electrons along the 1D path without grain boundaries and junctions, while hollow space can capture scattered light to increase light harvesting as well as easier separation and recovery than TiO2nanoparticles due to the length in the micrometer range[9].
Another strategy is to construct the heterostructures by the wide band-gap semiconductor with a narrow band-gap semiconductor(with the proper band positions)[1,10].Yu and Li fabricated and reported Agbased heterojunction[11-16],Au NPs loaded onto the α-Bi2O3/Bi2O2CO3[17],F-Bi2MoO6[18],anatase/rutileTiO2particles[19],and MoS2/CdS composite[20],which are more efficient than individual component in photocatalytic properties.Inparticular,QianandMagroups successfullyfabricatedUCNPs/semiconductorsfor NIR-driven photocatalysis,such as UCNPs/TiO2nanofiber[21],UCNPs/TiO2/CdS nanofibers[22],NYF@TiO2-Au core@shell microspheres[23].They show unique optical propertieswithwideabsorptionandenhanced photocatalytic abilities towards to organic dye removal efficiency under irradiation with NIR.Such synergistic interactions of heterojunction between two kinds of semiconductorsarefairlypowerfulnotonlyin improving the visible light harvesting ability but also in extending the lifetime of photoinduced electrons and holes via an internal charge transfer,facilitating the separation of electron-hole pairs and reducing the chance of recombination[24-26].
Among these,bismuth tungstate(Bi2WO6),as a typical Aurivillius oxide,has a layered structure with perovskite-like slabs of WO6and[Bi2O2]2+layer and has important physical and chemical properties such as ferroelectric piezoelectricity,catalytic behavior and nonlinear dielectric susceptibility[27-28].More importantly,Bi2WO6is a promising visible light-driven photocatalyst with high photocatalytic activity[29-30].However, the photocatalytic activity of pure Bi2WO6is limited by difficult migration and high recombination probability of photogenerated e--h+pairs.Therefore,the combination of tubular morphology and heterostructure construction is a useful approach for designing heterostructure photocatalysts with high charge separation efficiency.
In order to improve the photocatalytic activity, the construction of TiO2-Bi2WO6heterostructures has become a hot research,and some achievement has been obtained in recent years.For instance,Wang et al.successfully synthesized TiO2-Bi2WO6nanofibers by electrospinning technique[31].Colón et al.and other groups have reported TiO2modified flower[32]/sphere[33]/ hollow tube-like Bi2WO6[34].Wu and Luo et al.reported the preparation TiO2nanobelts[35]/TiO2nanotubes[36]grown on titanium(Ti)foil decorated with Bi2WO6nanocrystals,respectively.These results indicate that the photocatalytic activities of TiO2-Bi2WO6heterojunctions show enhanced photocatalytic performance in comparison with individual components of Bi2WO6or TiO2.To the best of our knowledge,much less notice has been taken of the preparation of TiO2nanotubes synthesized by alkali hydrothermal treatment modified with Bi2WO6.Moreover,few investigations were carried on the comparative mechanism of the enhanced photocatalytic activity for organic pollutants under multiple modes including UV,visible,and microwave-assisted photocatalysis.What is more,they lack direct evidence to explain photocatalytic mechanism under multiple modes that serve as background datafortheenvironmentalbehavioroforganic pollutants.
In this work,Bi2WO6/TiO2nanotubes(Bi2WO6/ TiO2-NTs)heterostructures were fabricated by multicomponent assembly approach combined with hydrothermal treatment,which is free from the usage of additives or surfactants.Subsequently,the photocatalytic activities of Bi2WO6/TiO2nanotubes under multiple modes including UV,visible,and microwave-assisted photocatalysis were also studied in this work.Direct evidencetoexplaincomparativelyphotocatalytic mechanism under multiple modes was supplied by free radical andholetrappingexperiments.The relationship between the morphology,structure,optical properties and the photocatalytic activities of Bi2WO6/ TiO2heterostructuresundermultiplemodeswas investigated in detail.
1 Experimental
1.1 Preparation of Bi2WO6/TiO2nanotubes
In a typical procedure[37],TiO2nanotubes were dispersed in H2O(5 mL)under vigorously stirring for 0.5 h.Meanwhile,Bi(NO3)3·5H2O(0.972 g)and Na2WO4·2H2O(0.329 g)were dissolved in glacial acetic acid (HAc,10 mL)and H2O(5 mL),respectively.Subsequently,the above solutions were added into TiO2nanotubes suspension to form a white suspension. After stirring for 2 h,the resulting mixture was suffered from hydrothermal treatment at 150℃for 4 h,and the resulting precipitate was dried and washed with deionized water for three times.The obtained powder was further dried at 80℃for 24 h.The final product was denoted as Bi2WO6/TiO2-NTs-x,where x representsthedopingofTiO2nanotubes(mass percentage).
1.2 Characterization of the catalyst
X-ray diffraction patterns were obtained on a Bruker-AXS(D8)X-ray diffractometer with Cu Kα radiation(λ=0.154 06 nm)at 40 kV and 40 mA in 2θ ranging from 20°to 80°.X-ray photoelectron spectroscopy(XPS)characterization was carried out on an ESCALAB 250Xi spectrometer equipped with Al Kα radiation at 300 W.N2adsorption-desorption isotherm analyses of samples were obtained at 77 K using Micromeritics3H-2000PS2.Themorphologiesof synthesized samples were analyzed using a scanning electron microscope(SEM)(HitachiS-4300)and transmission electron microscope(TEM)and high resolution transmission electron microscope(HRTEM)(JEM-2100F).UV-Vis diffused absorption spectra(UV-Vis DRS)were recorded using a UV-Vis spectrophotometer(TU-1901)over the wavelength range of 200~800 nm and BaSO4as a reference material.
1.3 Photocatalytic tests
Photocatalytic activities of the Bi2WO6/TiO2-NTs composite were studied by monitoring the degradation behaviors of rhodamine B(RhB)under multimode (including UV,visible,and microwave-assisted photocatalysis mode).The 125 W high pressure mercury lamp(λ=313.2 nm),400 W Xe lamp(λ=410.0 nm; moreover,the inner sleeve was made of No.11 glass to filter out ultraviolet from the Xe lamp),and 15 W microwave electrodeless lamp(MEL,UV emission wavelength mainly located at 278 nm,U shape,100 W output power of microwave reactor),were used as UV,visible light,and microwave-assisted photocatalysis mode light source,respectively.The concetration of RhB was 50 mg·L-1.Moreover,the amounts of the catalyst(liquid volume)for the three modes(UV, visible,and microwave-assisted photocatalysis)were 100 mg(100 mL),200 mg(220 mL),and 300 mg(500 mL),respectively.
The photocatalytic reaction was carried out in a quartz photoreactor.Prior to irradiation,the suspension containing the solid catalyst and an aqueous solution of the contaminant was ultrasonicated for 10 min and then stirred for 1.5 h in the dark to ensure adsorption-desorption equilibrium.The reaction temperature was maintained at(30±2)℃by circulation of waterthroughanexternalcoolingjacketorby circulating solution to a cooler with the peristaltic pump.At certain time intervals,suspensions(5 mL) were sampled and centrifuged to remove the photocatalyst particles.Decreases in the concentrations of RhB,methyl orange(MO),crystal violet(CV),and methylene blue(MB)were analyzed by TU-1901 UVVis spectrophotometer at λ=553,464,582,and 664 nm,respectively.
2 Results and discussion
2.1 Compositional and structural information
XRDwasusedtocharacterizethecrystal structure of the as-prepared Bi2WO6/TiO2-NTs,as well as pure TiO2-NTs and Bi2WO6(Fig.1).The diffraction peaks of pure TiO2-NTs and Bi2WO6are well matched with the standard patterns of anatase phase of TiO2(PDF No.21-1272)[37]andorthorhombicphaseof Bi2WO6(PDF No.39-0256),respectively.After the coupling of Bi2WO6and TiO2-NTs,when the TiO2-NTs loading increases from 25%to 50%,the diffraction peaks of TiO2intensify gradually,whereas the peak intensities of Bi2WO6decrease.No impurity peak is found in Bi2WO6/TiO2-NTs composites,suggesting that the composites exhibit a coexistence of both Bi2WO6and TiO2phases.
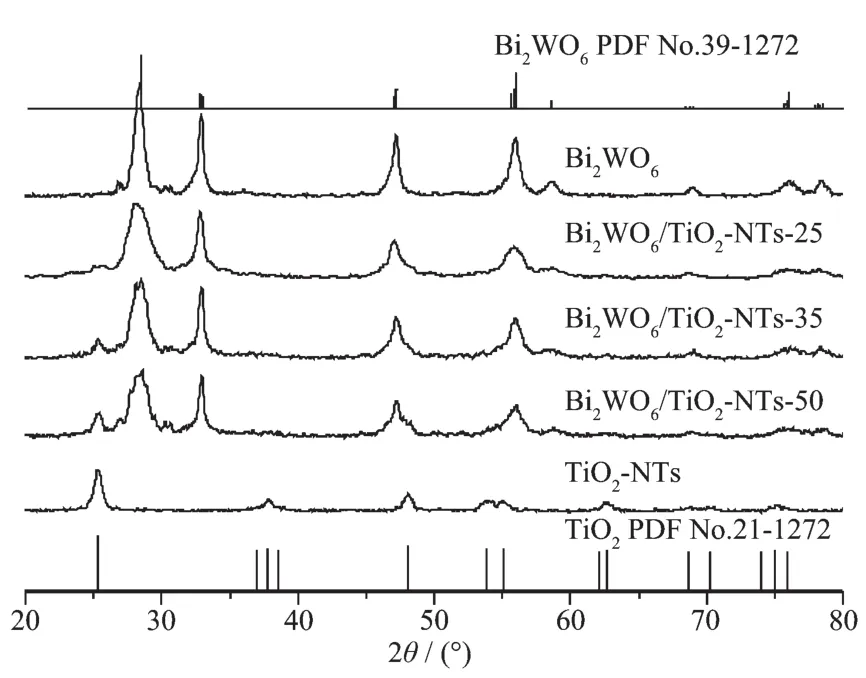
Fig.1 XRD patterns of the samples
Valence states and the surface chemical composition of the as-prepared samples were investigated by XPS technique.As shown in Fig.2a,the peaks at 458.68 and 464.48 eV are attributed to Ti2p3/2and Ti2p1/2,respectively,confirming the titanium species in the composite is Ti4+.After introduction of the Bi2WO6into the TiO2nanotubes,the binding energies of Ti2p3/2and Ti2p1/2shift to higher values(458.78 and 465.28 eV,respectively),which is attributed to diffusion of W6+ions into the TiO2lattice and further generation of WOTi bond linkage[35,37].As displayed in Fig.2b and c,for pure Bi2WO6,the characteristic peaks at 164.58 and 159.28 eV are ascribed to Bi4f5/2and Bi4f7/2from Bi3+in the lattice and the binding energy of W4f5/2and W4f7/2at 37.88 and 35.78 eV, respectively,are corresponded to W6+[35].In the XPS spectrum of Bi2WO6/TiO2-NTs,in contrast with Bi2WO6, the binding energy of Bi4f5/2(164.38 eV)and Bi4f7/2(159.08 eV)decreases by 0.2 eV while that of W4f5/2(37.58 eV)and W4f7/2(35.58 eV)decreases by 0.3 eV. The results suggest that the chemical environment surrounding Bi and W has changed,which is possiblyinfluenced by TiO2-NTs.Thus,we can confirm that the TiO2-NTs successfully modified by Bi2WO6.
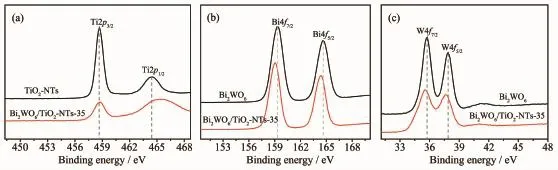
Fig.2 XPS spectra of Ti2p(a),Bi4f(b),and W4f(c)regions for TiO2-NTs,Bi2WO6,and Bi2WO6/TiO2-NTs-35
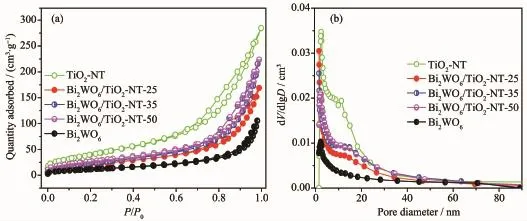
Fig.3 Nitrogen adsorption-desorption isotherms(a)and BJH pore size distribution curves(b)of samples
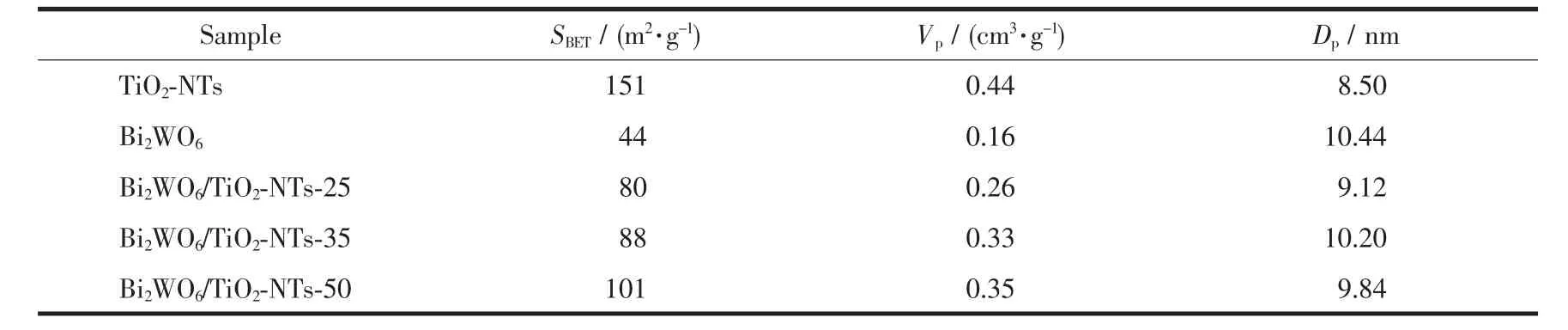
Table1 Textural parameters of various TiO2-based materials
The porosity of the Bi2WO6/TiO2-NTs heterostructures is investigated by N2adsorption-desorption isotherms and the corresponding BJH pore size distribution.As shown in Fig.3a,the isotherms exhibit typeⅣwith an H3 hysteresis loop characteristic of mesoporous material[37],which is confirmed by the pore size distribution(Fig.3b).Moreover,the formation of such mesoporous materials is attributed to the aggregation of the Bi2WO6nanoparticles adhering to the surface of the TiO2nanotubes.More importantly,as shown in Table 1,the measured BET surface areas of Bi2WO6/ TiO2-NTs-25(80 m2·g-1),Bi2WO6/TiO2-NTs-35(88 m2· g-1)and Bi2WO6/TiO2-NTs-50(101 m2·g-1)are greatly enhanced compared with that of Bi2WO6(44 m2·g-1). Meanwhile,the specific surface areas of composite materials increase indeed together with the increase of TiO2-NTs contents from 25%to 50%.
2.2 Morphology

Fig.4 SEM images of the samples
Themorphologyandmicrostructureofthe photocatalysts were also investigated.As shown in the SEM image(Fig.4a),TiO2-NTs show the nanotubular morphology with an average diameter of 30 nm andlength of 1 μm.While Bi2WO6exhibits a typical structure of nanosheet consist of nanoparticles with the side length of 50~250 nm and thickness of 20~40 nm(Fig.4b).As displayed in Fig.4c~e,morphologies of TiO2and Bi2WO6change obviously afterthe combination by TiO2-NTs and Bi2WO6through hydrothermal treatment.The typical morphology structure of Bi2WO6/TiO2-NTs-25 consists of smooth TiO2nanotubes and curled Bi2WO6flakes,which link mutually to each other.Moreover,the surface of TiO2nanotubes becomes rough obviously after Bi2WO6modification when TiO2nanotubes loading increases from 35%to 50%.While Bi2WO6changes from flakes to smaller nanoparticles.Furthermore,smaller Bi2WO6nanoparticles homogeneously disperse on the surface of TiO2nanotubes in-situ growth process.Compared with TiO2-NTs and Bi2WO6,aggregation of Bi2WO6/TiO2-NTs has intensively alleviated with the loading of TiO2nanotubes increasing from 0 to 50%.
In order to further confirm the Bi2WO6/TiO2-NTs heterostructures,HRTEM was used to investigate the detailed structure information of the Bi2WO6/TiO2-NTs. The corresponding HRTEM image displays two types of clear lattice fringes,as shown in Fig.4f.The interplanar spacing of 0.35 and 0.315 nm corresponds to the(101)crystal plane of TiO2-NTs and the(131) crystal plane of the orthorhombic phase of Bi2WO6, respectively[18-19].According to the results of XRD,XPS, SEM and HRTEM,we assume that Bi2WO6/TiO2-NTs heterostructures with Bi2WO6nanoparticles on the surfaceofTiO2nanotubeshavebeenprepared successfully.
Based on the above results and discussion,we put forward the plausible formation of Bi2WO6/TiO2-NTs heterojunction.Considering Bi(NO3)3with crystal water,Bi2O2(OH)NO3is formed through the following hydrolysis and condensation reaction in the glacial acetic acid-water system(Eq.1~2).When Na2WO4solution is added to the above reaction solution,Bi2WO6nanoparticles are obtained(Eq.3)[38].Then the introduction of TiO2-NTs into Bi2WO6suspension,Bi2WO6nanoparticles aggregate around TiO2-NTs.Subsequently,at high temperature and high pressure,Bi2WO6nanoparticlesgrowintocurledflakesorsmaller nanoparticles and homogeneously dispersed on the surface of TiO2nanotubes in-situ growth process,resulting in the formation of Bi2WO6/TiO2-NTs heterojunction[31].
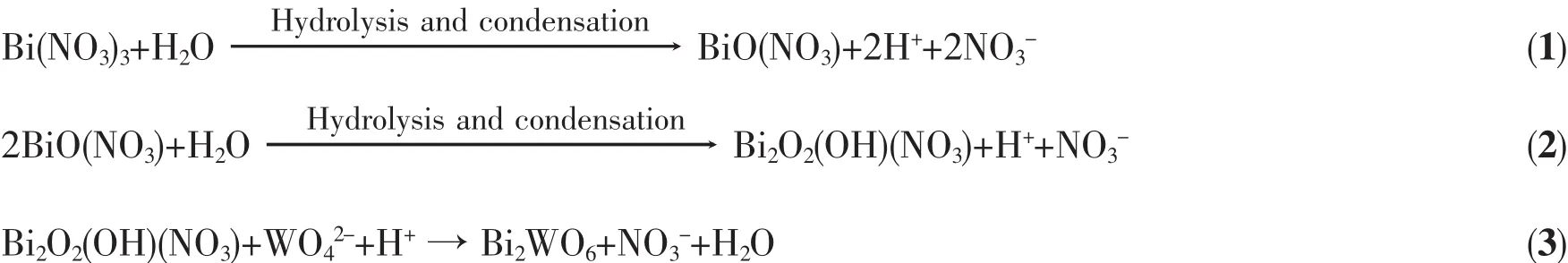
2.3 Optical property
UV-Visdiffusedabsorptionspectra(UV-Vis DRS)were carried out to investigate the optical properties of the photocatalysts.As shown in Fig.5a, the pure TiO2-NTs and Bi2WO6exhibit a fundamental absorption edge at around 388 and 450 nm,which originate from the charge transfer response of TiO2-NTs and Bi2WO6from the valence band to the conduction band,respectively[39].Compared with pure TiO2-NTs,the absorption edges of Bi2WO6/TiO2-NTs showed obvious red-shift to the longer wavelength within the range of visible light.
It is known that the optical absorption near the band edge of prepared samples obeys the following equation:(αhν)n=K(hν-Eg).In this equation,K,α,h, hν,Egare constant,absorption coefficient,Planck constant,energy of the incident photon,band gap, respectively,and n is 0.5 and 1 for a direct and indirect band gap semi-conductor[38].According to the formula, the calculated band gaps(Eg)of samples are 2.75 eV(Bi2WO6),2.87 eV(Bi2WO6/TiO2-NTs-25),2.94 eV (Bi2WO6/TiO2-NTs-35),3.00 eV(Bi2WO6/TiO2-NTs-50), and 3.20 eV(TiO2-NTs),respectively.
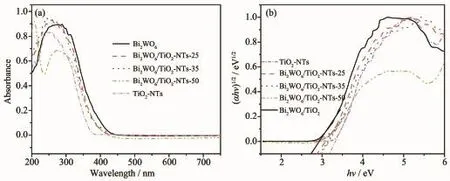
Fig.5 UV-Vis DRS(a)and plot of(αhν)1/2versus hν(b)for Bi2WO6,TiO2-NTs and the Bi2WO6/TiO2-NTs materials
The conduction band(CB)and valence band (VB)positions of the Bi2WO6and TiO2samples are estimated by the following equations:EVB=X-Ee+0.5Eg; ECB=EVB-Eg,where EVBand ECBare the VB and CB edge potentials,Eeis the energy of free electrons on the hydrogen scale(about 4.5 eV vs NHE).The X values for the Bi2WO6and TiO2materials are 6.21 and 5.81 eV,respectively[40-41].The Egof Bi2WO6and TiO2-NTs are estimated to be 2.75 and 3.20 eV,respectively. Herein,the CB and VB edge potentials of Bi2WO6and TiO2-NTs are calculated at 0.34 and 3.09 eV,and -0.29 and 2.91 eV,respectively.
2.4 Photocatalytic activity
The photocatalytic performance of the Bi2WO6/ TiO2-NTs heterostructures in terms of photodegradation of RhB molecules under multiple modes including UV,visible,and microwave-assisted photocatalysis was investigated.
Fig.6 ashowsthephotocatalyticactivitiesof photocatalysts.UnderUVlightirradiationalone (without catalyst),only 3%RhB is degraded,which means the RhB can remain stability under long time irradiation.However,apparent changes in the concentration of RhB are observed in the existence of both light and catalyst.After irradiation for 90 min,46.8%, 61.5%,70.0%,88.9%,82.4%and 74.1%of the RhB is degraded by using the TiO2-NTs,Bi2WO6,Bi2WO6/TiO2-NTs-25,Bi2WO6/TiO2-NTs-35,Bi2WO6/TiO2-NTs-50,and P25,respectively.
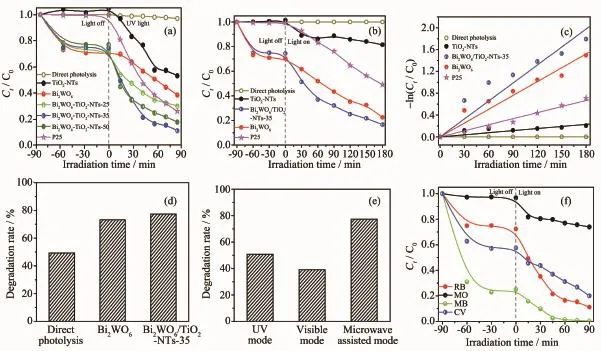
Fig.6 Normalized decrease concentration of Ct/C0of RhB solution containing different photocatalysts under UV(a) and visible(b)light irradiation;(c)-ln(Ct/C0)as a function of irradiation time for RhB degradation over photocatalysts;(d)Photocatalytic degradation RhB profiles obtained using different photocatalysts under microwave-assisted photocatalysis mode for 15 min;(e)Photocatalytic degradation RhB profiles by Bi2WO6/TiO2-NTs-35 obtained under multimode for 15 min;(f)Normalized decrease concentrations of Ct/C0of different dyes using Bi2WO6/TiO2-NTs-35 under UV light irradiation
Fig.6 b displays the photocatalytic activity of prepared samples under the visible light irradiation.It isfoundthatthephotocatalyticperformanceof Bi2WO6/TiO2-NTs-35 to degrade RhB under visible lightirradiationsurpassesthatofitsindividual counterparts.
At the same time,the kinetics of photocatalytic degradation of RhB is investigated by simplified Langmuir-Hinshelwood model.The pseudo-first-order rate constant(kapp)is calculated using the formula -ln(Ct/C0)=kappt,where C0and Ctare the initial concentration and concentration at reaction time t of RhB, respectively.From Fig.6c,under visible irradiation, the rate constant over Bi2WO6/TiO2-NTs-35,Bi2WO6, P25,and TiO2-NTs is 1.10×10-2,8.45×10-3,3.71×10-3, and 1.27×10-3min-1,respectively.Moreover,Bi2WO6/ TiO2-NTs-35 shows the highest first-order rate constant, which is about 1.2 and 8.7 times greater than that of pure Bi2WO6and TiO2-NTs,respectively.
Fig.6 d also exhibits the photocatalytic activity of differentphotocatalystsundermicrowave-assisted photocatalysis mode with electrodeless discharge lamp activated by microwaves as the light source.Bi2WO6/ TiO2-NTs-35showshighestphotocatalyticactivity towards RhB degradation under microwave-assisted photocatalysis mode.Moreover,Fig.6e displays photocatalytic activities of Bi2WO6/TiO2-NTs-35 under different modes after irradiation for 15 min.In contrast with UV and visible mode,the Bi2WO6/TiO2-NTs-35 shows higher activity under microwave-assisted photocatalytic mode.In addition,different kinds of dyes were selected to evaluate the photocatalytic activity under UV light irradiation(Fig.6f).The cationic dyes (CV,MB,and RhB)are effectively degraded,while the degradation of anionic dye(MO)is poor,which is attributed to the different structure and adsorption of dyes.
Toevaluatethestabilityandreusabilityof Bi2WO6/TiO2-NTs-35heterostructuresforpractical application,the photocatalytic degradation of RhB with the same photocatalyst is carried out for several times.As displayed in Fig.7,degradation curve has no obvious decline after four cycles of RhB degradation reaction under UV light irradiation,which indicates Bi2WO6/TiO2-NTs-35 heterostructures maintain high stability.
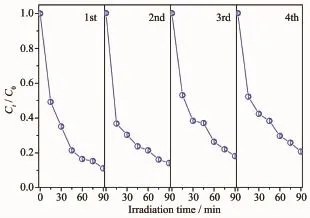
Fig.7 Recycling for the photodegradation of RhB in the presence of Bi2WO6/TiO2-NTs-35 under UV light irradiation
2.5 Possible pathway of RhB degradation in Bi2WO6/TiO2-NTs system
The above photocatalytic tests indicate that:(i) the photocatalytic activity of pure TiO2-NTs can be furtherincreasedbyintroductionproperBi2WO6loading under multimode;(ii)in contrast to UV and visible mode,Bi2WO6/TiO2-NTs showed higher photocatalyticactivityundermicrowave-assistedphotocatalysis mode.The influence factors towards the excellent photocatalytic activity of Bi2WO6/TiO2-NTs are discussed.
Firstly,Bi2WO6modified TiO2nanotubes play a major role in improving the photocatalytic activity of TiO2nanotubes.On one hand,according to UV-Vis DRS analysis,Bi2WO6/TiO2-NTs heterostructures have a narrow band gap and exhibit enhanced UV and visible light absorption,consequently increases the utilization of light.On the other hand,the formed heterostructuresbetweenBi2WO6andTiO2-NTs photocatalysts can extend the lifetime of photoinduced electrons and holes via an internal charge transfer, further facilitate the separation of e--h+pairs and reduce the chance of recombination.These wellseparated electrons and holes can participate in the overall photocatalysis process.
Secondly,the open mesoporous morphology of nanotubescan enhance the contactbetweenthe substance and photocatalysts during the photocatalytic reaction.Meanwhile,the nanotubes provide an efficient transport channel for photogenerated electrons.
Thirdly,degradation mode influences the photocatalytic activity of Bi2WO6/TiO2-NTs heterostructures. Compared with UV and visible mode,Bi2WO6/TiO2-NTs heterostructures display highest photocatalytic activity under microwave-assisted photocatalysis mode. Microwave enhances the reactants mobility and diffusion leading to increased exchange of reactants between catalyst surface and solution[42].Moreover,more·OH and·O2-radicals are generated by photocatalysis with microwave irradiation than photocatalysis alone to enhance the separation of e--h+pairs[43-45],which will be confirmed by the following trapping experiments.
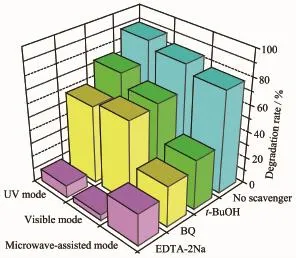
Fig.8 Controlled experiments using different radical scavengers for the degradation of RhB by Bi2WO6/TiO2-NTs-35 under different modes
As shown in Fig.8,the RhB degradation rate under UV degraded mode decreases obviously with the addition of disodium ethylenediaminetetraacetate (EDTA-2Na,1 mmol·L-1)as scavenger for h+(from 88.9%to 9.3%),is moderately reduced with the addition of benzoquinone(BQ,1 mmol·L-1)as scavenger for·O2-(from 88.9%to 58.6%)and tert-butyl alcohol (t-BuOH,1 mmol·L-1)as scavenger for·OH(from 88.9%to 73.7%)[46-48].Similar results are found in RhB photodegradationoverBi2WO6/TiO2-NTs-35under visible mode.Compared with UV and visible mode, under microwave-assisted mode,there isa little difference.The degradation rate toward RhB exhibits a significant decrease when EDTA-2Na,BQ,and t-BuOH are introduced.Furthermore,RhB degradation rate is reduced from the original 77.4%to 23.9%, 30.7%,and 37.5%,respectively.These results suggest that:(i)under the three modes,the degradation of RhB is primarily driven by h+,·OH,and·O2-;(ii) under the UV and visible mode,h+is the dominant reactive oxidants;(iii)under microwave-assisted mode, h+,·OH and·O2-make nearly equal contribution to RhB degradation.That is to say,more·OH and·O2-are generated under microwave-assisted photocatalysis mode compared with UV and visible mode.
Based on the above results,the photocatalytic mechanismforBi2WO6/TiO2-NTsheterostructures photocatalyst is tentatively proposed and schematically illustrated in Scheme 1.The conduction bands(CB) (the valence band(VB),band gap)of TiO2-NTs and Bi2WO6are at-0.29 and 0.34 eV,respectively. Hence,under UV or MEL irradiation,both TiO2-NTs and Bi2WO6are excited,and photogenerated electrons and holes are in their CB and VB,respectively. Subsequently,the photoexcited electrons in the CB of TiO2-NTs transfer to the CB of Bi2WO6,which is due to that ECBof TiO2-NTs is more negative than that of Bi2WO6.Simultaneously,the holes in the EVBof Bi2WO6move to TiO2-NTs due to that the EVBof Bi2WO6is more positive than that of TiO2-NTs.The hVB+reacts withtheabsorbedH2Omoleculesordeoxidizes dioxygen dissolved in the aqueous solution to form·OH radicals.In addition,the ECBcan be easily oxidized by dioxygen to produce·O2-radicals.With the help of·OH,hVB+and·O2-species,RhB is degraded and then mineralized.The photocatalytic process under visible light irradiation is similar with UV(microwave-assisted photocatalytic mode)except TiO2-NTs are not excited.

Scheme 1Photodegradation mechanism of Bi2WO6/TiO2-NTs-35 heterostructures photocatalyst under UV mode
3 Conclusions
In summary,Bi2WO6/TiO2-NTs heterostructures were fabricated by multicomponent assembly approach combined with hydrothermal treatment.Bi2WO6flakes or nanoparticles dispersed on the surface of TiO2nanotubes to form heterostructures.The prepared Bi2WO6/TiO2-NTs heterostructures exhibit considerably high photocatalytic activity towards the degradation of RhB under multimode including UV,visible and microwave-assistedphotocatalysis.Thisenhanced photocatalytic activity is due to more efficient separation of the e--h+pairs,originating from the introduction of Bi2WO6modified TiO2-NTs,the nanotubular geometries,and the degradation mode.The h+,·OH, and·O2-radicals are the main active species during the photocatalysis process under multimode.Moreover, more·OH and·O2-radicals are generated by photocatalyst with microwave-assisted irradiation.This work can provide important inspirations in developing the photocatalytic heterostructures materials.
Acknowledgments:Thisworkissupportedbythe Natural Science Foundation of China(Grants No.21376126, 81403067),the Program for Young Teachers Scientific Research in Qiqihar University(Grant No.2014K-M03),and the Basic Business Special Scientific Research of Heilongjiang Province Education Department(Grant No.135109204).
[1]Tian J,Zhao Z H,Kumar A,et al.Chem.Soc.Rev.,2014, 43:6920-6937
[2]Daghrir R,Drogui P,Robert D.Ind.Eng.Chem.Res.,2013, 52:3581-3599
[3]Sang L X,Zhao Y X,Burda C.Chem.Rev.,2014,114:9283-9318
[4]ZHANG Chao-Ying(張超穎),WANG Ping(王蘋),LIU Yan-Yan(劉巖巖),et al.Chinese J.Inorg.Chem.(無機化學學報),2017,33(7):1132-1138
[5]Murciano L T,Lapkin A A,Chadwick D.J.Mater.Chem., 2010,20:6484-6489
[6]Zhang Y L,Han C,Zhang G S,et al.Chem.Eng.J.,2015, 268:170-179
[7]Lee K,Mazare A,Schmuki P.Chem.Rev.,2014,114:9385-9454
[8]Wang X D,Li Z D,Shi J,et al.Chem.Rev.,2014,114:9346 -9384
[9]Wehrenfennig C,Palumbiny C M,Snaith H J,et al.J.Phys. Chem.C,2015,119:9159-9168
[10]Wang H L,Zhang L S,Chen Z G,et al.Chem.Soc.Rev., 2014,43:5234-5244
[11]Li J,Fang W,Yu C L,et al.Appl.Surf.Sci.,2015,358:46-56
[12]Yu C L,Zhou W Q,Yu J C,et al.Chin.J.Catal.,2014,35: 1609-1618
[13]Yu C L,Wei L F,Chen J C,et al.Ind.Eng.Chem.Res., 2014,53:5759-5766
[14]Yu C L,Li G,Kumar S,et al.Adv.Mater.,2014,26:892-898
[15]Yu C L,Wei L F,Zhou W Q,et al.Chemosphere,2016, 157:250-261
[16]Yu C L,Zhou W Q,Liu H,et al.Chem.Eng.J.,2016,287: 117-129
[17]Yu C L,Zhou W Q,Zhu L H,et al.Appl.Catal.B,2016, 184:1-11
[18]Yu C L,Wu Z,Liu R Y,et al.Appl.Catal.B,2017,209:1-11
[19]Zhang J,Xu Q,Feng Z C,et al.Angew.Chem.Int.Ed., 2008,47:1766-1769
[20]Zong X,Yan H J,Wu G P,et al.J.Am.Chem.Soc.,2008, 130:7176-7177
[21]Zhang F,Zhang C L,Peng H Y,et al.Part.Part.Syst.Char., 2016,33:248-253
[22]Zhang F,Zhang C L,Wang W N,et al.ChemSusChem, 2016,9:1449-1454
[23]Xu Z H,Quintanilla M,Vetrone F,et al.Adv.Funct.Mater., 2015,25:2950-2960
[24]Zhou F Q,Fan J C,Xu Q J,et al.Appl.Catal.B,2017,201: 77-83
[25]Zhang F,Wang W N,Cong H P,et al.Part.Part.Syst.Char., 2017,34(2):1600222(6 pages)
[26]Min Y L,He G Q,Xu Q J,et al.J.Mater.Chem.A,2014, 2:2578-2584
[27]Zhang L S,Wang H L,Chen Z G,et al.Appl.Catal.B, 2011,106:1-13
[28]ZHANG Tian(張田),ZOU Zheng-Guang(鄒正光),HE Jin-Yun(何金云),et al.Chinese J.Inorg.Chem.(無機化學學報),2017,33(6):954-962
[29]Huang J,Tan G Q,Ren H J,et al.ACS Appl.Mater.Interfaces,2014,6:21041-21050
[30]Zhang L W,Zhu Y F.Catal.Sci.Technol.,2012,2:694-706
[31]Zhang Y P,Fei L F,Jiang X D,et al.J.Am.Ceram.Soc., 2011,94:4157-4161
[32]López S M,Hidalgo M C,Navío J A,et al.J.Hazard.Mater., 2011,185:1425-1434
[33]Liu Z,Liu X Z,Lu D Z,et al.Mater.Lett.,2014,130:143-145
[34]Hou Y F,Liu S J,Zhang J H,et al.Dalton Trans.,2014,43: 1025-1031
[35]Li Y,Wu W J,Wu M Z,et al.Mater.Res.Bull.,2014,55: 121-125
[36]Deng F,Liu Y,Luo X B,et al.Sep.Purif.Technol.,2013, 120:156-161
[37]Ma F Y,Geng Z,Yang X,et al.RSC Adv.,2015,5:46677-46685
[38]Chen S F,Tang W M,Hu Y F,et al.CrystEngComm,2013, 15:7943-7950
[39]Di J,Xia J X,Ge Y P,et al.Appl.Catal.B,2015,168-169: 51-61
[40]Li L,Huang X D,Hu T Y,et al.New J.Chem.,2014,38: 5293-5302
[41]Dai K,Lu L H,Liang C H,et al.Appl.Catal.B,2014,156-157:331-340
[42]Zhang X W,Li G T,Wang Y Z.Dyes Pigm.,2007,74:536-544
[43]Genuino H C,Hamal D B,Fu Y J,et al.J.Phys.Chem.C, 2012,116:14040-14051
[44]Zhang Z H,Yu F Y,Huang L R,et al.J.Hazard.Mater., 2014,278:152-157
[45]Zhang X W,Sun D D,Li G T,et al.J.Photochem.Photobiol. A,2008,199:311-315
[46]Xiao J D,Xie Y B,Cao H B,et al.Catal.Commun.,2015, 66:10-14
[47]Lin S L,Liu L,Hu J S,et al.Appl.Surf.Sci.,2015,324:20-29
[48]Ma F Y,Shi T,Gao J,et al.Colloids Surf.A,2012,401:116-125
Comparison of Photocatalytic Activity of Bi2WO6/TiO2Nanotubes Heterostructures Composite under Multimode
MA Feng-Yan*,1YANG Yang1LI Na1YANG Qi-Lin1LI Shang-Jin2SHEN Lu-Yan2
(1College of Chemistry and Chemical Engineering,Qiqihar University,Qiqihar,Heilongjiang 161006,China)
(2College of Materials Science and Engineering,Qiqihar University,Qiqihar,Heilongjiang 161006,China)
Bi2WO6/TiO2nanotubes(Bi2WO6/TiO2-NTs)heterostructures composite were synthesized by multicomponent assembly approach combined with hydrothermal treatment employed TiO2nanotubes as template.Multiple techniques such as X-ray powder diffraction(XRD),X-ray photo-electron spectroscopy(XPS),N2adsorptiondesorption,scanning electron microscopy(SEM),high resolution transmission electron microscopy(HRTEM),and UV-Vis diffused absorption spectra(UV-Vis DRS)were applied to investigate the composition,structures, morphologies,optical and electronic properties of as-prepared samples.The heterostructures were formed with Bi2WO6nanoflakes or nanoparticles attached on the surface of TiO2nanotubes.The photocatalytic activity of Bi2WO6/TiO2-NTs heterostructures was evaluated sufficiently by photodegradation of rhodamine B(RhB)under multimode including UV,visible and microwave-assisted photocatalysis.Compared to TiO2nanotubes and Bi2WO6,Bi2WO6/TiO2-NTs-35 shows the highest photocatalytic activity under multimode.In contrast with UV, visible mode,the Bi2WO6/TiO2-NTs-35 shows the highest activity toward RhB degradation under microwaveassisted photocatalytic mode.This enhanced photocatalytic activity is due to the more efficient separation of thee--h+pairs,originating from the introduction of Bi2WO6modified TiO2-NTs,the nanotubular geometries,and degradation mode.The main active species of the degradation process are proven to be h+,·OH,and·O2-radicals. Moreover,more·OH and·O2-radicals were generated under microwave-assisted photocatalytic mode.
TiO2nanotubes;Bi2WO6;multimode degradation;photocatalysis
TB33
A
1001-4861(2017)09-1656-11
10.11862/CJIC.2017.199
2017-04-18。收修改稿日期:2017-07-18。
國家自然科學基金(No.21376126,81403067)、齊齊哈爾大學青年教師科學技術類科研啟動支持計劃項目(No.2014K-M03)和黑龍江省教育廳基本業務專項理工面上項目(No.135109204)資助。
*通信聯系人。E-mail:mafengyan989@163.com

