Effect of environmental stresses and high hydrostatic pressure on the Antibiotic susceptibility of pathogenic Vibrioparahaemolyticus
Oulimata Ndiaye, LIU Hai-quan,2,3, PAN Ying-jie,2,3, ZHAO Yong,2,3*
(1.College of Food Science and Technology, Shanghai Ocean University, Shanghai 201306;2. Shanghai Engineering Research Centerof Aquatic-Product Processing & Preservation,Shanghai 201306;3. Laboratory of Quality & Safety Risk Assessment for Aquatic Products on Storage and Preservation (Shanghai), Ministry of Agriculture, Shanghai 201306)
Effect of environmental stresses and high hydrostatic pressure on the Antibiotic susceptibility of pathogenicVibrioparahaemolyticus
Oulimata Ndiaye1, LIU Hai-quan1,2,3, PAN Ying-jie1,2,3, ZHAO Yong1,2,3*
(1.CollegeofFoodScienceandTechnology,ShanghaiOceanUniversity,Shanghai201306;2.ShanghaiEngineeringResearchCenterofAquatic-ProductProcessing&Preservation,Shanghai201306;3.LaboratoryofQuality&SafetyRiskAssessmentfor
AquaticProductsonStorageandPreservation(Shanghai),MinistryofAgriculture,Shanghai201306)
The aim of the present study was to determine the effect of environmental stresses (temperature, pH, osmotic pressure, high hydrostatic pressure (HHP)) on the antibiotic susceptibility of 12 different pathogenicVibrioparahaemolyticusisolates. Isolates were subjected to growth at (30 ℃and 37 ℃), an osmotic pressure of (1% and 6% NaCl), a media at pH (6.0 and 9.0) and three different HHP treatment (180, 250, 300 MPa). The minimal inhibitory concentrations (MICs) of tested antibiotics used against unstressed (control), stressed or post-stressed isolates were determined using the broth microdilution method. The study found that incubation under increased salt (6%), reduced salt (1%) and increased pH (9.0) conditions were commonly associated with increased antibiotic resistance. Incubation at 30 ℃ temperature, reduced pH (6.0) and HHP treatment were commonly associated with decreased antibiotic resistance. Besides, both ciprofloxacin and cefotaxime kept a constant MIC during almost all stress challenges. Thus our data demonstrate that exposure ofV.parahaemolyticusto some stress conditions may contribute on the rapid development of antibiotic resistance in this food borne pathogen.
PathogenicVibrioparahaemolyticus; Antibiotic susceptibility; Environmental stresses; HHP
Environmental stress can be defined as an external factor that has a negative impact on the physiological welfare of bacterial cells causing a reduction in growth rate and dramatically, an inhibition or death. These bactericidal stresses can be listed as extremes of temperature, osmotic pressure, pH, nutrient depletion[1], and also the use of toxic or inhibitory compounds like antibiotics[2]. During food processing bacteria get through a variety of conditions that may lead to chemical (acids, ethanol, alkaline, chlorine and salts) and physical (heat, radiation and pressure) stresses[3]. In this context, it is interesting to note that high hydrostatic pressure (HHP) can also inactivate microorganisms. In the food industry area, HHP is known to be the best alternative for thermal process in order to maintain the nutritional and functional values of food[4-7]. However, it has been shown that some cells can repair the sub-lethal damage induced by HHP, allowing them to proliferate once they have healed from the injury. Stress-inhibited bacteria act to retaliate the impact of environmental stresses by making phenotypic and genotypic adaptations[8]. Phenotypic alterations, including expression of protective shock proteins allow resistance to subsequent challenge with the same stress (stress hardening)[9], and thus are able to lead to cross-protection against a range of foreign challenges notably resistance to antibiotics[10-13]. It is currently believed that bacterial cells sense the actions of antibiotics just like any other form of environmental stress[2]. The mar (multiple antibiotic resistance) operon regulates the expression of a large variety of genes, including those coding for at least one broad-specificity efflux pump (the arcAB efflux pump), which are more strongly expressed under environmental stress conditions[14-16]. Stress alike oxidative stress, pH extremes, heat shock, osmotic shock, starvation, are known to be causing bacterial genomic reorganization or mutation[17], and such mechanisms of adaptive mutation have been related with the development of ABR[18]. This implies a direct linkage between environmental stresses happening in food industry and domestic environment[14], and the development of antibiotic resistance[19-20].Vibrioparahaemolyticusis part of the expanding group of water and food-borne pathogens. It is an important pathogen of shrimp, widely spread in the aquatic environment and increasingly causing significant economic problems within the aquaculture industry[21-22]. The factors ofV.parahaemolyticusmajor virulence are the thermostable direct hemolysin and the thermostable direct hemolysin-related hemolysin, encoded respectively the by thetdhandtrhgenes. The association between possession of thetdhgene by a strain and its faculty to cause gastroenteritis has been proved[23]. In the aquaculture field, the presence of pathogenicvibriosin shrimp ponds has brought farmers to look for effective control methods such as antibiotic therapy. Studies indicate that the antibiotic resistance ofvibriosisolated from penaeid culture environment is quite common[24-25], a fact that apparently constitutes a problem to this type of aquaculture activity[26]. In addition, there is the risk of environmental impact, since the use of antibacterial agents as prophylactic measure in aquaculture favors the selection of resistant bacteria, increasing the probability of transferring resistant genes to human pathogens and land animals[27]. A huge number of antibiotic resistant genes found in bacteria and environments can be listed as follows, β-lactam and penicillin resistant genespenA andblaTEM-1[28-29], chloramphenicol resistant genescatI,catII,catIII,catIV, andfloR[30], tetracycline resistant genestetA,tetB,tetC,tetD,tetE,tetG,tetH,tatJ,tatY,tatZ, and many more[31-33]. Via conjugation, transduction, or transformation, these antibiotic resistant gene can be transferred among different bacteria[34]. Antimicrobial resistant pathogenicV.parahaemolyticusis increasing exponentially and is expected to create a serious problem in the clinical treatment[35].Recently there have been some useful studies done to evaluate the change of antimicrobial susceptibility after exposure to certain stress and results nourish even more our concern and curiosity in that topic. Meng-Hsuan Lin et al[36]investigated the Susceptibility ofVibrioparahaemolyticusto disinfectants after prior exposure to sublethal stress. In our laboratory, mismatch between antimicrobial resistance phenotype and genotype of pathogenicV.parahaemolyticusisolated from seafood has been published[37]. Considering the importance of the increasing antibiotic-resistant bacteria in marine invertebrates intended for human consumption, this study aimed to determine the antibiotic susceptibility pattern of pathogenicV.parahaemolyticusstains isolated from sea food after exposure to different stress conditions.
1 Materials and Methods
1.1 Materials
Bacterial strains As shown in Table 1, a total of 12 pathogenicV.parahaemolyticusisolates were used in this study. Two were American Type Culture Collection , ATCC 17802, ATCC 33847, and 10 were isolated from 4 types of seafood (Macrobrachiumnipponensis,PenaeusMonodon,PenaeusVannamei,Crassostreagigas), collected from wholesale markets during 2009 to 2013inShanghai.ThepresumptiveV.parahaemolyticusisolates were confirmed by the API 20E system (bioMérieux, Inc., Durham, NC, USA). Since previous reports have shown that the API 20E test was unreliable for the identification of theV.parahaemolyticusisolates[38-39], all strains were identified by the presence of the species-specifictlhgene using polymerase chain reaction (PCR) according to Kaysner and DePaola[40].
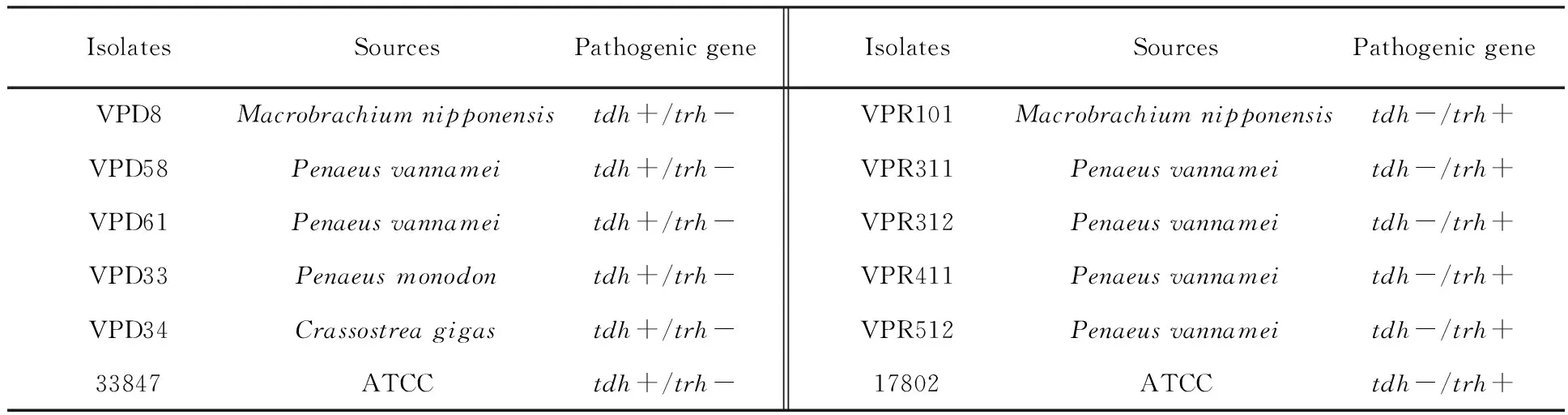
Table 1 Sources and genotypes of pathogenic Vibrio parahaemolyticus isolates (n=12)
1.2 Methods
1.2.1 Preparation of antibiotic stock solutionsV.parahaemolyticusisolates were treated with 7 antibiotics (Table 2), commonly used in clinical therapy. These antimicrobials belongs to 3 classes which are: β-lactam (Amoxicillin-clavulanic acid: AMC, Cefotaxime: CTX), aminoglycoside (Amikacin: AK, Gentamincin: GN, Kanamycin: K, Streptomycin: S), quinolone (Ciprofloxacin: CIP). Antibiotic stock solutions were prepared following the manufacturer recommendations. All antibiotic stock solutions were sterilized using 0.20 mm disposable syringe filter units (ToyoRoshi Kaisha, Ltd., Tokyo, Japan) and serially diluted to provide the desired concentrations (0.01-1 000 μg/mL) in the reaction mixtures.
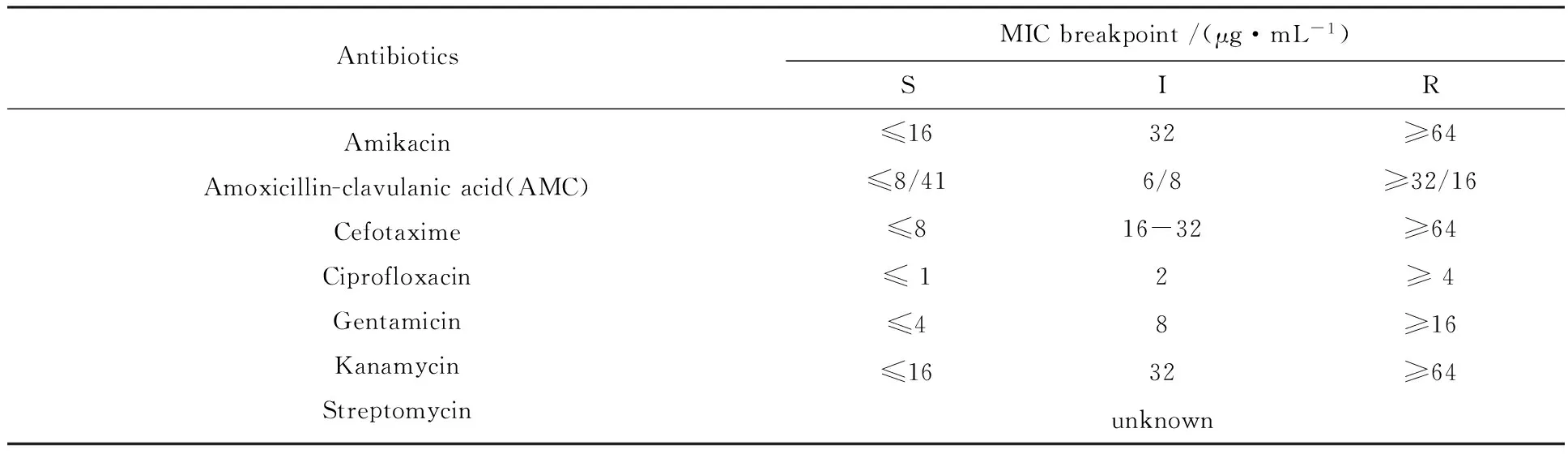
Table 2 Minimal inhibition concentration (MIC) breakpoints used to determine antibiotic susceptibility[41]
1.2.2 Preparation of unstressed (control) and stressed (temperature, pH, NaCl)V.parahaemolyticuscultures UnstressedV.parahaemolyticusisolates were cultured in Tryptic Soy Broth (Sigma Aldrich, France) supplemented with 3% NaCl at 37 ℃ for 18 h until they reached the stationary phase. The pH for that standard TSB solution was 7.5.V.parahaemolyticusstrains exposed to a low temperature stress were grown into a standard TSB solution. However the incubation temperature was 30 ℃ for 18 h. To examine the effect of post-low temperature condition on the susceptibility ofV.parahaemolyticustoward tested antibiotics, cells initially stressed by a growth at 30 ℃ were inoculated again into a standard TSB and incubated at 37 ℃ for 18 h. Two different levels of NaCl were used in TSB for the osmotic stress: 1% and 6% (wt/vol). The NaCl-supplemented suspensions were inoculated withV.parahaemolyticusand incubated at 37 ℃ for 18 h. Two different levels of pH were also used in the current study: 6.0. and 9.0. These pH Values were obtained by adding appropriate volumes of NaOH or HCL to TSB.V.parahaemolyticuscells grown from the different culture conditions were harvested by centrifugation (Herolab, UniCen M, Wiesloch, Germany) at 3 000 g for 10 min and pellets were resuspended and diluted to give a final concentration of approximately 5 log cfu/mL in the reaction mixtures for the antibiotic challenge.
1.2.3 High hydrostatic pressure treatment Three different isolates were used for the HHP treatment:Macrobrachiumnipponensis(VPD8),Peanusmonodon(VPD33) andCrassostreagigas(VPD34). Strains were cultured in Tryptic Soy Broth (Sigma Aldrich, France) supplemented with 3% NaCl at 37 ℃ for 18 h until they reached stationary phase. Resulting cell suspensions were aseptically transferred to sterile polyethylene bags (internal length, 13 mm, and width, 6 mm; Yizuo Co., LTD., Taiwan) in 8 mL portions, vacuum packaged, and the bags were heat-sealed immediately. Assessment of the HHP treatment was conducted using a laboratory high-pressure processing system with a 2-L vessel (HHP. L2-600/2; Tianjin Sai Mei Int. Trade Co., Ltd, Tianjin, China). The bags containing samples were subjected to the pressure treatment in triplicate 180, 250 and 300 MPa, with a holding time of 5 min at room temperature around 25 ℃. After processing, the cultures treated with HHP were aseptically removed from the bags. Cultures were diluted to give a final concentration of approximately 5 log cfu/mL in the reaction mixtures for antibiotic challenge as described above. Cell suspensions were challenged by antibiotic exposure as described below.
1.2.4 Antimicrobial assays (broth microdilution method) The minimal inhibitory concentration (MIC), defined as the lowest concentration of antibiotic required to inhibit visible growth of the test microorganisms, was determined using the broth micro-dilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines[41]EscherichiacoliATCC 25922 was included as a quality control strain. To confirm the MIC, a 100 mL sample was taken at the end of incubation and viable cell numbers in visibly clear wells were assessed by plating on TSA. The MIC was confirmed if the viable cell numbers after incubation were not significantly different from the numbers at initial inoculation. The number of survivors was determined by plating on TSA before and after exposure to stress. Each antibiotic sensitivity trial was performed in triplicate. The breakpoint MICs of tested antibiotics againstV.parahaemolyticuswas interpretedaccordingto methods recommended by the CLSI[41].
1.2.5 Statistical analysis Statistically significant differences (P<0.05) were established between control between control and test data were evaluated as recommended by Mann-Whitney U analysis using the Statistical Package for the Social Sciences (SPSS), version 11.0.
2 Results
2.1 The antibiotic resistance of unstressedVibrioparahaemolyticus
All unstressed isolates (Table 3) used in this research were initially sensitive toward the antibiotics However, differential susceptibility was observed according to the type of antibiotic and also between isolates.

Table 3 Changes in MIC of antibiotics applied to low temperature stressed V. parahaemolyticus strains
Note (same as table four, five and six):“+” 0.5 to 2 fold increase in MIC (P<0.05);“-” 0.5 to 2 fold decrease in MIC (P<0.05);“++” 2.1 to 4-fold increase in MIC (P<0.05);“--” 2.1 to 4-fold increase in MIC (P<0.05);“+++”greater than 4-fold increase in MIC (P<0.05);“---” greater than 4-fold decrease in MIC (P<0.05);0 no change (P> 0.05);PS: post stress
All strains showed the same susceptibility to cefotaxime and ciprofloxacine.Penaeusvannameiisolate(VPR512) and both,tdh,trhfrom commercial ATCC were more sensitive to gentamicin and amikacin. ATCC strains showed the higher sensitivity to amoxillin-clavicul acid (AMC) while themacrobrachiumnipponensisisolate (VPR101) and theCrassostreagigasisolate (VPD34) were less sensitive toward it. As a matter of fact this finding indicates different MIC susceptibility leaded with either the type of antibiotic or the type of isolate. The highest degree of inhibition was obtained when samples were treated with cefotaxime and ciprofloxacin. More than 90% of the isolates showed a growth inhibition after treatment with these two antibiotics in any of the applied stress conditions.
2.2 The antibiotic resistance of temperature stressed and post-temperature stressedVibrioparahaemolyticus
At 30 ℃, an increase in antibiotic susceptibility was noticed (Table 3). The MIC value was reduced especially for the streptomycin and kanamycin. However, the majority ofV.parahaemolyticusisolates had a MIC similar to those observed in the unstressed or control suspensions. Those antibiotics involve gentamicin, amikacin and particulary amoxillin-clavicul acid.Crassostreagigasisolate (VPD34) had lower MIC of amoxillin-clavicul acid. Further, this susceptibility decreased or remained constant after removal of the stress.
2.3 The antibiotic resistance of pH stressedVibrioparahaemolyticus
An increase in susceptibility was noticed at pH 6.0 (Table 4). Isolates exposed to amoxillin-clavicul acid and streptomycin was found to be less resistant at pH 6.0. Toward the rest of the antibiotics they showed a constant resistance and occasionally became less resistant. In contrast the resistance of the isolates raised up at pH 9.0 specially face to amikacin.Peanusmonodonisolate (VPD33) became resistant with a MIC raised up to 2 fold toward that antibiotic. Globally, the resistance increased or remained constant at pH 9.0.

Table 4 Changes in MIC of antibiotics applied to acid-stressed V. parahaemolyticus strains
4.2 預約優(yōu)選和排序功能 輸液時段設計是減少患者等候,均衡工作量的一個關鍵環(huán)節(jié)。我們廣泛聽取患者的意見和建議,總結手工預約的實踐經(jīng)驗,常規(guī)狀態(tài)下系統(tǒng)每天設置7個時間段,每50分鐘為1個時間段,2個時間段之間有10 min的緩沖期,每個時間段預約50例患者。根據(jù)患者喜好和輸液高峰時段的情況,系統(tǒng)默認為患者選擇最早的時段,其中10:30~11:20時段只留給當日就診后需要輸液的患者預約,也可以根據(jù)患者的需求自由選擇時間段。系統(tǒng)根據(jù)患者預約的先后為每個時段的患者自動排序。智能化的操作非常簡便、高效和人性化。這一功能的實現(xiàn) 也是“關愛患者,從細節(jié)做起”的體現(xiàn)。
2.4 The antibiotic resistance of osmotic stressedVibrioparahaemolyticus
Osmotic stressedV.parahaemolyticusisolates grown at 1% NaCl showed an increase of antibiotic resistance although with some isolate-antibiotic pairs, no significant change was detected (Table 5).
2.5 The antibiotic resistance of post HHPVibrioparahaemolyticus
An increase of antibiotic susceptibility was found according to the applied pressure treatment (Table 6). With a pressure of 180 MPa there was no significant difference between treated and untreatedV.parahaemolyticusisolates. However, a significant increase in susceptibility was noticed when the pressure was raised to 250 MPa.
This was further increased when isolates wassubjected to 300 MPa at the same holding time which was 5 mn in all cases. For example, with a treatment at 180 MPaCrassostreagigasisolate (VPD34) had the same susceptibility as the control strain. Eventually its susceptibility increases by 2 fold at 250 MPa, and by 4 fold at 300 MPa. Nevertheless for some isolates-antibiotics couples, no change occurred after the HHP treatment. That was the situation of all isolates treated with gentamicine and theMacrobrachiumnipponensisisolate (VPD8) when treated with kanamycine.

Table 5 Changes in MIC of antibiotics applied to osmotically stressed V. parahaemolyticus strains
Note: A significant increase of resistance up to 2 fold was revealed when isolates such asPeanusmonodon(VPD33) andCrassostreagigas(VPD34) were treated by amikacin. Similar results were observed in isolates grown at 6% NaCl but the increase was expressed at comparatively lower levels in samples grown at 1% NaCl

Table 6 Changes in MIC of antibiotics applied toV. parahaemolyticus strains survivors from HHP treatment
3 Discussion
Traditionally,vibriospecies are considered highly susceptible to the vast majority of antimicrobials[42]. However, antimicrobial resistance was reported in the recent decades and has evolved since, due to the unreasonable use of antimicrobials products in almost all systems reliable to living species[43-44]. An antibiotic might be expected to have variable effects on culture of genetically susceptible bacteria when the growth conditions allow different growth rates. Very few studies have addressed the impact of environment on the antibiotic susceptibility ofVibrioparahaemolyticus. To our knowledge, this is the first study noticing antibiotic resistance differences ofVibrioparahaemolyticusexposed to different kinds of stress. Nonetheless a number of studies have observed the differences in overallVibrioparahaemolyticusgrowth because of varying culture temperature[45-48]. For example, in bacteriological broth systems the growth ofV.parahaemolyticusin a temperature range between 8 to 45 ℃[49]and the growth rate and lag time from 10 to 30 ℃ were modeled[50]. In the current study, all strains were at first selected to be initially sensitive toward the antibiotics tested. When analyzing our results according to the antibiotic mechanism, we could notably observe that bacterial susceptibility was not influenced by the antibiotic class. Bacterial susceptibility toward ciprofloxacin and cefotaxime were very high compared to other antibiotics. However the mechanism of these bactericides is different, the cefotaxime act by the inhibition of bacterial cell wall synthesis while the Ciprofloxacin act by the inhibition of Protein Synthesis. The high sensitivity ofVibrioparahaemolyticustoward these two antibiotics was previously reported by Chan et al[51]. For the group of aminoglycoside antibiotic, they didn’t either give the same susceptibility pattern. Results were different from each antibiotic itself in all the stress conditions. Our study revealed an increase in antibiotic susceptibility ofV.parahaemolyticusin some cases when the organism was subjected to low temperature. A similar response has been reported inE.coli,S.entericaserovarTyphimurium, andS.Aureusgrown in conditions of continuing low-temperature stress by McMahon et al[52]. It has also been reported that increased antibiotic susceptibility was not maintained inE.colicultures when low-temperature stress was removed, nor inS.entericaserovarTyphimuriumorS.aureuscultures[52]. These temporary changes might be due to transient alterations affecting the bacterial cell wall or cell membranes. Modification of a normal environmental temperature can has adverse effect on the membrane fluidity. Consequently bacteria will make compensatory alterations in the degree of saturation of membrane fatty acids[53-56]to reestablish ideal states of membrane fluidity. However, more studies are needed to explore the process by which thermal stress can induce persistent increases in antibiotic susceptibility. In further support of the present observations, Salomon et al[8]found that T6SS1, highly regulated systems used by Gram-negative bacteria has an anti-bacterial activity under 23 ℃ and 30 ℃, but not at 37 ℃ in both high salt (MLB media) and low salt (LB media) conditions. Interestingly these findings closely match our results which showed in some cases a decrease of antibiotic resistance at 30 ℃. A high anti-Hcp response was also previously detected in cystic fibrosis patients with chronicP.aeruginosainfections[57], suggesting that Hcp is available for immune processing during bacterial infection. Furthermore, by reverting to our results, the minority of cases showing an increase of susceptibility at 30 ℃ might be explained if we relay to the fact that the expression of T6SS1 in free-living bacteria is minimal[58]. To our knowledge, that is the first evidence linking a component of a T6S system to antibiotic resistance. Even though the resistance mechanism is still a blur, it probably involves uncharacterized effectors of this secretion system.
Ecological stresses such as osmotic, acid and cold shock provoke mutation in bacterial cells that has been reported to be related with the emergence of antibiotic resistance[18]. Under conditions of high salt, low salt or high pH stresses, the MICs of the antibiotic applied to most of our isolates were higher than the MICs of unstressed suspensions. McMahon et al[52]obtained the same results when the antibiotic resistance ofE.colisuspension was challenged in conditions of high-salt. However in contrast to our results in whichV.parahaemolyticuscells displayed a decrease of resistance, McMahon et al[52]reported an increase of resistance when the pH value was reduced. An increase greater than two fold was noticed in two isolates (VPD33 and VPD34) grown in medium with 1% NaCl and treated by amikacin. For example, isolates stressed at 1% NaCl showed in increase greater than that shown for isolates stressed at 6% NaCl. The resistance was also more pronounced with the couple, isolate (VPD33) and amikacin which showed once again an increase up to 2 fold at pH 9.0. In further support of the present observations, McMahon et al[52]found that an osmotic pressure more than 4.5% NaCl increased the antibiotic resistance ofE.coli,SalmonellaTyphimuriumandS.aureus. In addition, Ganjian et al[59]found that NaCl concentrations up to 35% augmented the antibiotic resistance ofS.aureuscells.
Reaction face to acid stress is an intricate phenomenon. Acid stress can be described as the combined biological effect of low pH and weak (organic) acids, such as acetate, propionate and lactate present in the environment (food) as a result of fermentation, or alternatively, when added as preservatives[60-62]. Exposure to acid stress may enhance bacterial pathogenesis by altering the virulence-associated characteristics of acid adapted cells. The reduced cytotoxicity of acid-adapted cell could be a consequence of physical injury.V.choleraecells grown at pH 6.0 had reduced expression of various enzymes necessary for lipopolysaccharide biosynthesis, leading to deterioration in the external membrane and an increased susceptibility to hydrophobic drugs like erythromycin[63].
In our study, an increase of susceptibility was noticed following the pressure treatments (180 MPa, 250 MPa) and became more dramatic when the applied pressure was 300 MPa. Damages from HHP treatment with pressure level setting similar to ours is well documented in the literature. HHP causes inhibition of protein synthesis and reversible protein denaturation at 50 MPa-100 MPa, membrane damage at 200 MPa, and irreversible protein denaturation at ≥300 MPa[64-66]. These results demonstrated increasing changes in cell morphology with increasing pressure treatment. To our knowledge, the present study is the first to assess the effects of post HHP treatment on the antibiotic susceptibility of microorganisms. The inactivating effects of HHP may be facilitated by the presence of antimicrobial agents[45]. Because Bacteriocins do require a given incubation time to cause cell damage their bactericidal effect in combination with an HHP treatment are more pronounced Some studies have been reported in the combination of antimicrobial and HHP treatments of lower intensity (500 MPa, 5 min). The inactivation of staphylococci in pudding by nisin improved when combined with HHP. Viable counts reductions significantly increased by 0.87 log cycle cycle for the 500 UI/g nisin-HHP combination comparing to the single HHP treatment. Nisin was again tested singly or in combination with HHP treatment (500 MPa, 5 min) for staphylococci in pudding during one week storage at 4 ℃. The single addition of nisin dramatically (P<0.005) reduces viable counts of staphylococci, in rice pudding[67].
Another research has found that the addition of antibiotics to the medium that should be used for HHP treatment can improve the disinfection efficacy of HHP treatment. Firstly, a variety of bacteria were isolated from the ossicles. Irrespective of the medium, HHP treatment at 350 MPa for 10 minutes gave satisfying but incomplete elimination especially of Gram-negative bacteria. The addition of antibiotics increased the efficacy of the inactivation[45].
This part of the study was to evaluate ifV.parahaemolyticuscells that have survived HHP treatment could develop antibiotic resistance or become more susceptible.V.parahaemolyticusselected in this study, did not develop any antibiotic resistance after the stress from HHP treatments. The MICs of the antibiotics applied to each isolates were lower than the MICs for (control) unstressed organisms depending on the applied pressure. Our findings suggest also that combination of HHP and antibiotics could improve the efficacy of HHP treatment. Despite the fact that only three samples were used for each of the combinations of antibiotics and HHP, conclusions regarding the superiority of one combination to one over could also be drawn from this study.
This study made it possible to investigate various effects of stress on the antibiotic susceptibility ofV.parahaemolyticuscells. It was found that incubation under some type of stress (30 ℃ and reduced pH) may increase bacterial susceptibility while exposure to some others (increased pH, increased and reduced salt) may have the adverse effect. In general, the decrease in antibiotic resistance at low temperature stress was not maintained after removal of the stress. Our research showed that no increase of resistance was found in the antibiotic susceptibility testing after HHP treatments. This suggests that exposure ofV.parahaemolyticusto certain stress conditions may contribute on the rapid development of antibiotic resistance in this food borne pathogen. However more studies are warranted to validate these results and further understand the mechanisms underlying variations in antibiotic susceptibility.
Reference
國家自然科學基金面上項目(31271870, 31571917);上海水產(chǎn)品加工及貯藏工程技術研究中心(11DZ2280300);
Oulimata Ndiaye 女,碩士研究生。研究方向為食品安全。 E-mail: olitaah@live.com
環(huán)境壓力條件與超高壓對致病性副溶血性弧菌耐藥性的影響
Oulimata Ndiaye1, 劉海泉1,2,3, 潘迎捷1,2,3, 趙 勇1,2,3*
(1.上海海洋大學 食品學院,上海 201306;2.上海水產(chǎn)品加工及貯藏工程技術研究中心,上海 201306;3.農業(yè)部貯藏保鮮質量安全風險評估實驗室(上海),上海 201306)
分析不同環(huán)境壓力條件(溫度、pH、滲透壓和超高壓)對12株致病性副溶血性弧菌耐藥性的影響。利用微量肉湯稀釋法測定菌株在不同溫度(37 ℃和30 ℃)、滲透壓(1%和 6% NaCl)、pH(6.0和9.0)及超高壓(180、250和300 MPa)條件影響下,其對所測抗生素的最小抑菌濃度(MIC)。結果表明,在1%和6% NaCl質量分數(shù)、pH 9.0條件下,副溶血性弧菌的耐藥性增強,而在30 ℃、pH 6和超高壓條件影響下,其耐藥性減弱。此外,菌株在所測環(huán)境壓力條件影響下,其對環(huán)丙沙星和頭孢類抗生素的耐藥性基本保持不變。……

