Preparation, crystal structure and electrochemistry properties of a isopolytungstate Na10[H2W12O42]·20H2O·2HCOOH
LIU Yajie, YIN Baoli, CHEN Lijuan
(Henan Key Laboratory of Polyoxometalate Chemistry, College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China)
?
Preparation, crystal structure and electrochemistry properties of a isopolytungstate Na10[H2W12O42]·20H2O·2HCOOH
LIU Yajie, YIN Baoli, CHEN Lijuan*
(HenanKeyLaboratoryofPolyoxometalateChemistry,CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China)
A pure inorganic isopolytungstate Na10[H2W12O42]·20H2O·2HCOOH (1) has been synthesized in aqueous solution and its structure is characterized by IR spectrum and single-crystal X-ray diffraction. The X-ray diffraction reveals that 1 belongs to the triclinic space groupP-1 and consists of one [H2W12O42]10-polyanion, ten Na+cations, twenty lattice water molecules and two dissociative HCOOH molecules. The [H2W12O42]10-polyanion in 1 contains four tri-tungsten-oxo clusters linked to each other via corner-sharing mode. It is worth noting that the four tri-tungsten-oxo clusters can be divided into two pairs: two of them are located in the equatorial position of 1 with the formula {W3O14}, and the other are positioned at polar position, which can be formulated as {W3O13}. Moreover, the electrochemistry properties of 1 have been investigated at room temperature.
isopolytungstate; aqueous solution; electrochemistry
As a family of emblematic anionic metal-oxygen clusters, polyoxometalates (POMs) have attracted a high level of attention, not only due to abundant electronic configurations and bonding patterns in sophisticated structures but also their potential applications in magnetism catalysis, medicine, gas sorption, nonlinear optics and electronic materials[1-10]. As is known to all, POMs are usually categorized into two main types: heter-polyoxometalates (HPOMs) and iso-polyoxometalates (IPOMs). Among them, the design and synthesis of novel HPOMs have become a research hotspot which may be attributed to the following reasons: 1) steric configuration of heteroatoms is conductive to stabilize POMs’ structure; 2) prepared HPOMs (such as [B-α-SbW9O33]9-[11], [A-α-GeW9O34]10-[12], [A-α-HAsW9O34]8-[13]) can work as excellent precursors to fabricate more complex POM derivatives. Nevertheless, the investigations on IPOMs attract much less attention despite IPOMs bearing a number of features, which may be greatly the fact that the mechanism of formation of POMs is not well understood. To date, only a handful of IPOMs with neoteric structures and charming properties have been documented. For example, in 2008, Cronin et al. communicated an inorganic crown-shaped [H12W36O120]12-[14], an “S”-shaped [H4W22O74]12-, and a “§”-shaped [H10W34O116]18-[15]. Afterwards, KORTZ et al. reported aV-shaped [H2W28O95]20-that consists of two undecatungstate {W11} fragments and a hexatungstate fragment {W6}[16]. What’s more, our group also devoted great effort to the design of novel IPOMs. In 2014, we reported two series of oxalate-connective IPOMs {[Ln2(C2O4)(H2O)4(OH)W4O16]2}10-and {[Ln(C2O4)W5O18]4}20-(Ln = EuIII, HoIII, ErIII, or TbIII)[17]. Subsequently, we discovered three types of diverting IPOMs chain-like {[Ln4(H2O)22W28O94H2]2}12-[Ln = Pr3+, Nd3+, Sm3+], two-dimensional [Eu(H2O)7]2[Eu(H2O)5]2[W22O74H2]2-and discrete [Ln(H2O)4] [Ln(H2O)5]2[W22O74H2]5-[Ln = Gd3+, Tb3+, Er3+, Tm3+, Yb3+, Lu3+][18]. As a part of our continuous exploitation on IPOMs, herein, we obtained another IPOT Na10[H2W12O42]·20H2O·2HCOOH (1) (CCDC 1537787) by utilizing the conventional aqueous solution method. Furthermore, the electrochemical properties of 1 were discussed.
1 Experimental
1.1 Physical measurements
All purchased chemicals were used without further purification. The IR spectrum was obtained by a solid sample pelleted with KBr on Nicolet FT-IR360 spectrometer in the range of 4 000-400 cm-1. Cyclic voltammogram (CV) measurements were performed on a CS310 electrochemical workstation at room temperature. A conventional three electrode system was used. The glassy carbon was employed as a working electrode. Ag/AgCl was used as a referencing electrode and platinum electrode was worked as a counter electrode. Moreover, the scan rate was 30-200 mV/s and the CV curves of 1 were carried out in Na2SO4+ H2SO4buffer solution.
1.2 Synthesis of 1
Na2WO4·2H2O (2.430 g, 7.368 mmol) and H2C2O4(0.063 g, 0.700 mmol) were dissolved in 15 mL water and stirred for 0.5 min. The pH value of the solution was carefully adjusted to 7.00 using a dilute HCl solution (6 mol/L). The solution was heated with stirring about 10 min, cooled to room temperature and filtered. Slow evaporation of the filtrate at room temperature led to massive colorless lump crystals several days later. Yield: ca. 35% (based on H2C2O4).
1.3 X-ray crystallography
The intensity data for 1 were acquired at 296(2) K using a Bruker APEX-II CCD diffractometer (Mo Kαradiation,λ= 0.071 073 nm, graphite monochromator), which corrected for routine Lorentz polarization as well as for SADABS program. Its structure was solved directly and refined by utilizing full-matrix least-squares methods with the SHELXTL-97 program package[19]. H atoms attached to O atoms were geometrically placed. No H atoms concatenated with water molecules were located. All H atoms were refined isotropically as a riding mode using the default SHELXTL parameters. The crystallographic data and structure refinements are shown in Table 1.
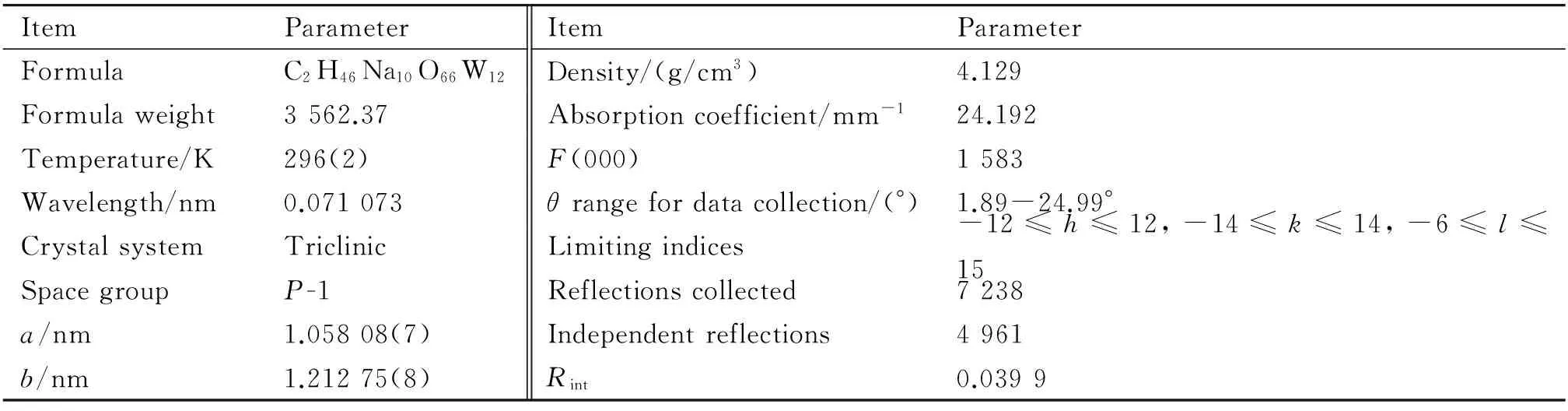
Table 1 Crystal data and structural refinements of 1
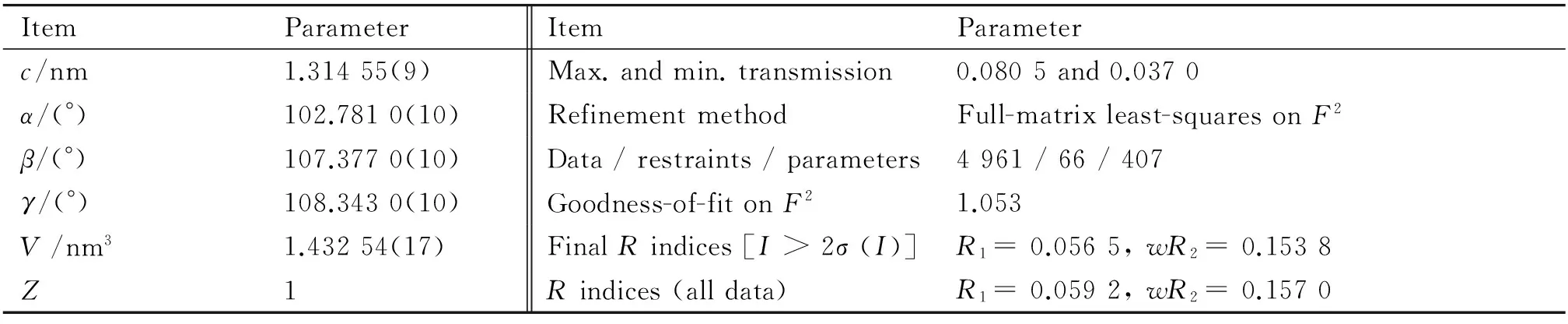
Continued to Table 1
2 Results and discussion
2.1 Description of crystal structure
The molecular structural unit of 1 consists of one [H2W12O42]10-polyanion (Fig.1a), ten Na+cations, twenty crystallization water molecules and two HCOOH molecules. Single-crystal X-ray diffraction shows that 1 crystallizes in the triclinic space groupP-1. In the structure of 1, six-coordinate W centers exhibit the octahedral coordination environments with W-O distances of 0.171 57(14)-0.229 34(14) nm, which is consistent with other reported POMs[20-21]. As we all know, in the classical Keggin-type structure (Fig.1b), the central atom displays a tetrahedral coordination geometry defined by fourμ4-oxygen atoms from four triangular {W3O13} units, and all W centers exhibit the octahedral coordination environment. In each {W3O13} triad, three WO6octahedra are fused together in the edge-sharing mode. For example, the plenary [α-SiW12O40]4-anion consists of a central SiO4group with four corner-sharing {W3O13} triads[22-23]. However, in the prime molecular structural unit of 1, the four {W3} triads are not identical to each other. Two of them are the triangular {W3O13} segments (Fig.1c) which are antisymmetric arranged on the polar positions of 1. And the other two triads are the crescent-shaped {W3O14} segments (Fig. 1d) taking up the equatorial position of the whole framework in a symmetrical mode. It is also of interest that the adjacent [H2W12O42]10-units are combined together through Na-O-Na bridges giving rise to a 3-D architecture (Fig.2).
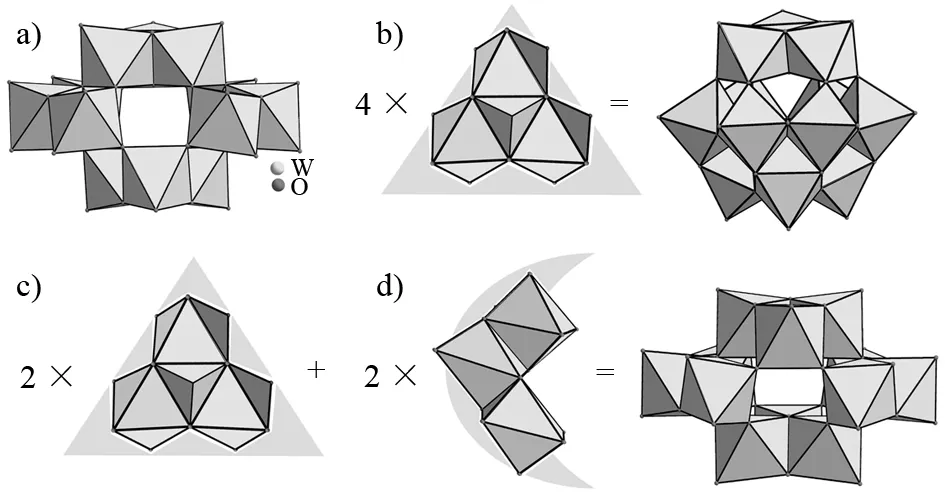
Fig.1 (a) Structure unit of 1, (b) The {W3O13} cluster in Keggin-typed structure, (c) The {W3O13} cluster in the polar position of 1, (d) The {W3O14} cluster in equatorial position of 1
2.2 IR spectrum
The IR spectrum of 1 has been collected by using KBr pellet between 4 000 and 400 cm-1(Fig.3). It is obvious that the occurrence of the vibration band at 3 381 cm-1implies the presence ofν(O-H) stretching mode of lattice water molecules[12,17]. In the low-frequency domain, the IR spectrum exhibits the characteristic vibration patterns resulting from the IPOT fragments. The strong peak in 935 cm-1can be assigned to the characteristic vibration band of the W=O bonds, while the strong peaks in 870 and 723 cm-1are attributed to theν(W-O-W) stretching vibrations[18]. Moreover, the middle peak located at 1 624 cm-1is indicative of the occurrence of HCOOH molecules in 1. In a word, the result of IR spectrum is in good agreement with single-crystal structural analysis.
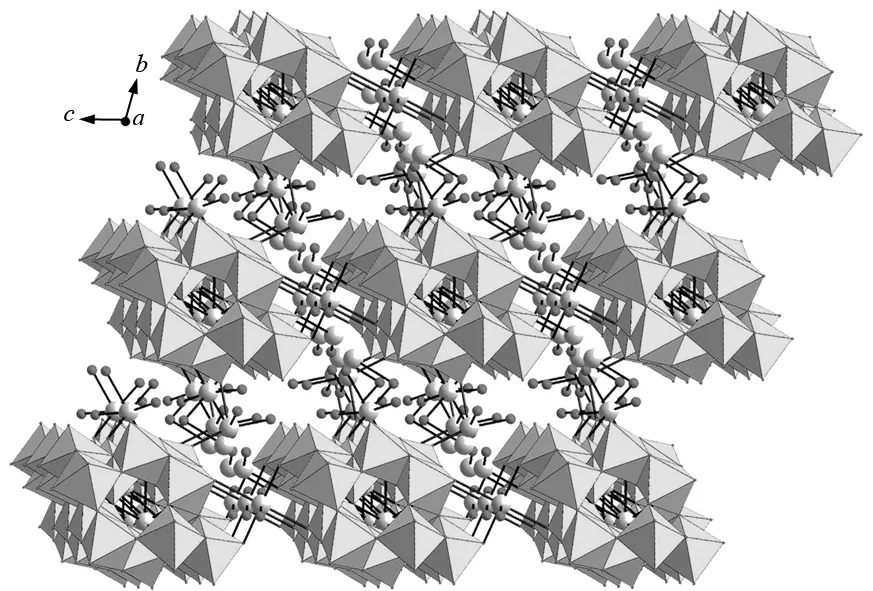
Fig.2 3-D packing structure of 1
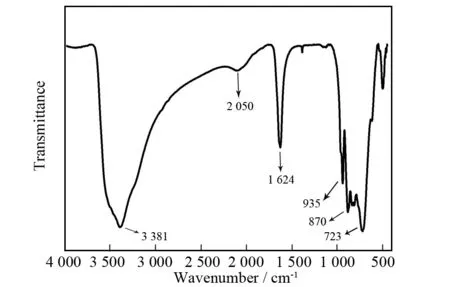
Fig.3 IR spectrum of 1
2.3 Electrochemistry property
Much attention and efforts have been put into the electrochemical studies of POMs since TOTH and ANSON reported the electrochemistry of POMs in 1989[24], which may be attributed to that POMs as electron reservoirs can deliver electrons to other species in the redox processes[25]. Hence, cyclic voltammetry (CV) experiment was performed to examine the redox properties of 1 in 0.5 mol/L Na2SO4+ H2SO4aqueous solution at room temperature. As is known, the electrochemical behavior can be affected by the pH value[22,26]. Thus, we select the pH value of 4.00 (Fig.4a) as the best choice by making a comparison of the CV curves in different pH values. Furthermore, as is shown in Fig.4b, at a scan rate of 30 mV/s, two pairs of redox waves withE1/2= -1.140 V(I/I′) andE1/2= -0.518 5 V (II/II′) [E1/2= (Epa+Epc)/2, vs Ag/AgCl electrode] in the negative potential direction can be observed in the potential range from -1.3 V to 0.9 V. The two redox peaks I/I′ and II/II′ correspond to the redox processes of W centers of polyoxoanion[7]. At the same time, the peak potential separations (ΔEp) of three couples of redox waves (I-I′, II-II′) in the cyclic voltammograms are 29 mV and 79 mV, respectively, which indicates the quasi-reversible two-electron charge-transfer (I-I′) and one-electron charge-transfer (II-II′) redox procedure of WVIcenters[7].
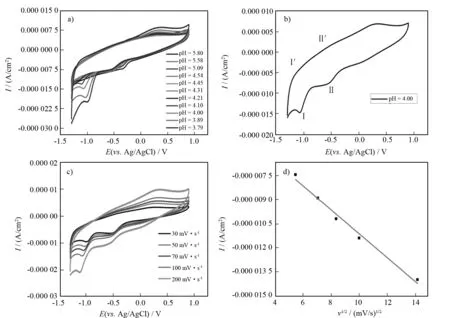
Fig.4 (a) Variation of cyclic voltammograms of 1 (concentration: 5 × 10-4 mol/L) at different pH values. Scan rate: 100 mV/s. (b) Cyclic voltammogram of 1 (concentration: 5 × 10-4 mol/L) in 0.5 mol/L Na2SO4 + H2SO4 aqueous solution (pH = 4.00). Scan rate: 30 mV/s. (c) Cyclic voltammograms of 1 at different scan rates (concentration: 5 × 10-4 mol/L) (d) Variation of cathodic peak currents of the WVI-based wave (I/I′) with different scan rates (30, 50, 70, 100, 200 mV/s)
We also investigated the influence of the scan rate on the electrochemical property of 1, and the scan rates were changed from 30 to 200 mV/s. It is very evident that the cathodic peak potentials shift toward the negative direction while the corresponding anodic peak potentials to the positive direction as scan rate increased (Fig.4c). Additionally, with the increase of the scan rates from 30 to 200 mV/s, the peak current intensities (Ipc) are proportional to the square root of the scan rates and the linear equation isIpc= -7.48×10-7ν1/2- 3.65×10-7with the correlation coefficient of 0.984 (Fig. 4d), indicating that the electrode reaction is controlled by diffusion process.
3 Conclusion
In summary, a purely inorganic IPOT Na10[H2W12O42]·20H2O·2HCOOH has been synthesized in aqueous solution and characterized by IR spectrum, single-crystal X-ray diffraction and electrochemistry. We will explore the appropriate reaction conditions so that multi-carboxylic acid ligand and transition metal ions can be introduce to the system to manufacture novel IPOMs with excellent properties. We believe that more intriguing IPOMs will be found in the near future.
[1] RITCHIE C, REBER C, BOSKOVIC C, et al. Sensitization of lanthanoid luminescence by organic and inorganic ligands in lanthanoid-organic-polyoxometalates [J]. Inorganic Chemistry, 2012, 51(2): 1142-1151.
[2] SHI D Y, SHANG S S, ZHAO J W, et al. Three novel 2D organic-inorganic hybrid CuII-LnIIIheterometallic arsenotungstates [J]. Synthetic Metals, 2012, 162(11/12): 1030-1036.
[3] MAL S S, KORTZ U. The wheel-shaped Cu20tungstophosphate [Cu20Cl(OH)24(H2O)12(P8W48O184)]25-ion [J]. Angewandte Chemie International Edition, 2005, 44(24): 3777-3780.
[4] FUKAYA K, YAMASE T. Alkali-metal-controlled self-assembly of crown-shaped ring complexes of lanthanidez/[α-AsW9O33]9-: [K{Eu(H2O)2(α-AsW9O33)}6]35-and [Cs{Eu(H2O)2(α-AsW9O33)}4]23-[J]. Angewandte Chemie International Edition, 2003, 42(6): 654-658.
[5] LUO J, MA X, CHEN L J, et al. Synthesis, structure and properties of an organic-inorganic hybrid independent 1-D double-chain Keggin-type silicotungstate with mixed ligands [J]. Inorganic Chemistry Communications, 2015, 54: 25-30.
[6] NIU J Y, WANG J P, WANG K H, et al. Assembly chemistry between lanthanide cations and monovacant Keggin polyoxotungstates: two types of lanthanide substituted phosphotungstates [{(α-PW11O39H)Ln(H2O)3}2]6-and[{(α-PW11O39)Ln(H2O)(η2,μ-1,1)-CH3COO}2]C [J]. Crystal Growth and Design, 2009, 9(10): 4362-4372.
[7] ZHAO J W, SHI D Y, CHEN L J, et al. Tetrahedral polyoxometalate nanoclusters with tetrameric rare-earth cores and germanotungstate vertexes [J]. Crystal Growth and Design, 2013, 13(10): 4368-4377.
[8] ZHANG P P, PENG J, PANG H J, et al. An interpenetrating architecture based on the Wells-Dawson polyoxometalate and AgI interactions [J]. Crystal Growth and Design, 2011, 11(7): 2736-2742.
[9] WANG X L, LI Y G, ZHANG Y, et al. Polyoxometalate-based porous framework with perovskite topology [J]. Crystal Growth and Design, 2010, 10(10): 4227-4230.
[10] SHA J Q, PENG J, ZHANG Y, et al. Assembly of multiply chain-modified polyoxometalates: from one- to three-dimensional and from finite to infinite track [J]. Crystal Growth and Design, 2009, 9(4): 1708-1715.
[11] WANG J P, NIU J Y, MA P T, et al. A novel organic-inorganic hybrid based on tungstoantimonate: synthesis, crystal structure, and properties of Na[{Cu(2,2′-bpy)(H2O)}2{Cu(2,2′-bpy)}2(B-α-SbW9O33)]·2H2O [J]. Chemistry Letters, 2006, 35(9): 994-995.
[12] ZHANG Z M, QI Y F, QIN C, et al. Two multi-copper-containing heteropolyoxotungstates constructed from the lacunary Keggin polyoxoanion and the high-nuclear spin cluster [J]. Inorganic Chemistry, 2007, 46(20): 8162-8169.
[13] ZHAO J W, WANG J P, CHEN L J, et al. Hydrothermal syntheses and structural characterization of two sandwich-type arsenotungstates [J]. Journal of Coordination Chemistry, 2010, 63(12): 2042-2055.
[14] STREB C, MCGLONE T, BRüCHER O, et al. Hybrid host-guest complexes: directing the supramolecular structure through secondary host-guest interactions [J]. Chemistry-A European Journal, 2008, 14(29): 8861-8868.
[15] MIRAS H N, YAN J, CRONIN L, et al. Structural evolution of “S”-shaped [H4W22O74]12-and “§”-shaped [H10W34O116]18-isopolyoxotungstate clusters [J]. Angewandte Chemie International Edition, 2008, 47(44): 8420-8423.
[16] KORTZ U, ISMAIL A H, BASSIL B S, et al. Synthesis and structural characterization of the 28-isopolytungstate fragment [H2W28O95]20-stabilized by two external lanthanide ions [Ln2(H2O)10W28O93(OH)2]14-[J]. European Journal of Inorganic Chemistry, 2009(34): 5247-5252.
[17] ZHAO J W, LI H L, LI Y Z, et al. Rectangle versus square oxalate-connective tetralanthanide cluster anchored in lacunary Lindqvist isopolytungstates: syntheses, structures, and properties [J]. Crystal Growth and Design, 2014, 14(11): 5495-5505.
[18] LI H L, YANG W, WANG X H, et al. Self-assembly of a family of isopolytungstates induced by the synergistic effect of the nature of lanthanoids and the pH variation in the reaction process: syntheses, structures, and properties [J]. Crystal Growth and Design, 2016, 16(1): 108-120.
[19] SHELDRICK G M. SHELXTL-97: Program for crystal structure solution [CP]. G?ttingen: University of G?ttingen, 1997.
[20] MA X, SONG K F, CAO J, et al. Synthesis, structure and electrochemical properties of a FeIII-CeIIIheterometallic sandwich-type tungstoantimonate with novel 2-D infinite structure [Ce(H2O)8][Ce(H2O)6] [Fe4(H2O)10(B-β-SbW9O33)2]·16H2O [J]. Inorganic Chemistry Communications, 2015, 60: 65-70.
[21] YANG W, LI H L, LI Y Y, et al. Syntheses, structures and properties of a series of 3s-5d-4f mixed metal substituted sandwich-type arsenotungstates [J]. Inorganic Chemistry Communications, 2015, 60: 71-76.
[22] YANG G Y, ZHAO J W, LI B, et al. A banana-shaped iron (III)-substituted tungstogermanate containing two types of lacunary polyoxometalate units [J]. Inorganic Chemistry Communications, 2009, 12(2): 69-71.
[23] BOND A M , ZHANG J, TAN W-T, et al. Mechanistic analysis of the electrocatalytic properties of dissolvedαandβisomers of [SiW12O40]4-and solid [Ru(bipy)3]2[α-SiW12O40] on the reduction of nitrite in acidic aqueous media [J]. Inorganic Chemistry, 2006, 45(9): 3732-3740.
[24] WANG X, WANG E, LAN Y, et al. Renewable PMo12-based inorganic-organic hybrid material bulk modified carbon paste electrode: preparation, electrochemistry and electrocatalysis [J]. Electroanalysis, 2002, 14(1): 15-16.
[25] AMMAM M, EASTON B E. Selective determination of ascorbic acid with a novel hybrid material based 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid and the dawson type ion [P2Mo18O62]6-immobilized on glassy carbon [J]. Electrochimica Acta, 2011, 56(7): 2847-2855.
[26] SU Z M, WANG X L, CHEN W C, et al. pH-Controlled and sulfite anion-directed assembly of a family of cerium(III)-containing polyoxotungstates clusters [J]. Inorganic Chemistry, 2014, 53(18): 9486-9497.
[責任編輯:劉紅玲]
同多鎢酸Na10[H2W12O42]·20H2O·2HCOOH的制備、晶體結構及其電化學性質
劉雅潔,殷保麗,陳利娟*
(河南省多酸化學重點實驗室,河南大學 化學化工學院,河南 開封 475004)
在水溶液中合成了一種純無機的同多鎢酸鹽Na10[H2W12O42]·20H2O·2HCOOH(1),并通過紅外光譜和X射線單晶衍射對其結構進行了表征. X射線單晶衍射表明1屬于三斜晶系P-1空間群,由1個[H2W12O42]10-聚陰離子、10個Na+離子、20個游離的水分子和2個游離的甲酸分子組成. 1中的[H2W12O42]10-聚陰離子由4個以共角方式相連的三金屬鎢氧簇構成. 值得注意的是這4個三金屬鎢氧簇可以被分成兩組:其中2個位于赤道位置,可以描述為{W3O14};另外2個三金屬鎢氧簇則處于極位,可以描述為{W3O13}. 此外,我們在室溫下對其電化學行為進行了研究.
同多鎢;水溶液;電化學
Supported by the Natural Science Foundation of China (21671054, U1304208) and the Students Innovative Pilot Plan of Henan University (16NA005).
, E-mail:ljchen@henu.edu.cn.
O614.3 Document code: A Article ID: 1008-1011(2017)03-0301-06
Received date: 2017-01-03.
Biography: LIU Yajie (1995-), female, majoring in polyoxometalate-based functional materials.*