貝伐單抗維持治療腦轉移瘤療效與安全性分析
石大友+廖立瀟+熊超
摘要:目的 評估貝伐單抗治療全腦放療后復發或進展腦轉移瘤的臨床療效。方法 回顧分析放療后進展或復發腦轉移瘤患者21例,共計28處病灶。患者有原發腫瘤病史且均取得病理,病灶均同時滿足以下各項影像學證據:CT或MRI增強病灶表現為灶內強化且伴有明顯水腫影;所有患者均給予貝伐單抗5 mg/kg,每14 d重復1次,治療4~8個周期。每4周期治療后第5周期治療前均行MRI檢查,比較治療前后T1WI相增強病灶大小變化及T1WI相病灶水腫變化。記錄患者治療前、后臨床癥狀、kps評分改變情況,治療前、后病灶大小、水腫變化以及KPS評分行t檢驗。結果 21例患者均安全完成治療,未見明顯嚴重不良反應。自第1周期治療結束患者臨床癥狀明顯改善,4周期后kps評分平均提高13.8分。MRI T1WI增強相可見強化區域病灶較治療前平均縮小54.8%,(P=0.001),MRI T2WI相可見水腫區域較治療前平均縮小80.7%(P=0.004)。結論 初步證實貝伐單抗能控制腦轉移瘤,明顯減輕顱內水腫,并改善腦轉移瘤的臨床癥狀。
關鍵詞:貝伐單抗;腦轉移瘤;顱腦水腫;全腦放療
Abstract:Objective To evaluate the clinical effect of bevacizumab for recurrence or progression brain metastases after whole brain radiotherapy .Methods Retrospective analysis of 20 cases of recurrence or progession brain metastases after radiotherapy, a total of 28 lesions. Patients had primary tumor history and pathology were obtained, the lesions were also satisfy the following the image evidence: CT or MRI enhanced lesions showed foci in the strengthening and accompanied by edema; All patients were given bevacizumab 5mg / kg, every 14 days repeated 1 time for 8 cycles. After the forth cycles of treatment, MRI was examined before and after treatment, and the changes of T1WI phase and edema of T1WI phase were compared before and after treatment. The clinical symptoms, KPS score , the size of the lesion, edema were record respectively before and after treatment. Comarision before and afer treatment was perofomed by paried t test. Results All the 20 patients receive treatment successfully, and no serious adverse reactions were found. The clinical symptoms of the patients were significantly improved after first cycles of treatment, and the KPS score increased by 13.8 points on average after 4 cycles. The regional lesions of T1WI MRI enhanced phase were smaller average 54.8% than before treatment, (P= 0.001), T2WI MRI edema region were smaller average 80.7% compared with the before treatment (P= 0.004).Conclusion Bevacizumab is preliminary confirmed to control the brain metastatic tumor, alleviate cerebral edema, and improve clinical symptoms of brain metastases.
Key words:Bevacizumab; Brain metastases; Encephaledema;Whole brain radiotherapy
惡性腫瘤腦轉移是腫瘤常見的并發癥,約20%~40%的癌癥患者發生腦轉移,尤其是好發生于肺癌和乳腺癌患者[1]。發生腦轉移患者預后極差,自然生存時間1~3個月,全腦放療(whole brain therapy,WBT)中位生存時間延長至3~6個月。故50年來全腦放療一直是腦轉移癌的標準治療手段。但是全腦放療有效率60%~80%,多數腫瘤短暫緩解后再次進展,中位生存期4~6個月,1年生存率14%~20%[2-3]。且再次復發后患者生活質量受到嚴重影響,治療手段有限,常規再次放療易造成正常神經組織壞死,預后極差。VEGF是目前發現的最重要的促進腫瘤血管生成的因子。在腫瘤的生長、復發、轉移以及改變血腦屏障的通透性有重要的作用[4-6]。文獻報道,晚期腫瘤患者外周血VEGF水平明顯高于早期肺癌,VEGF可刺激腫瘤組織中微血管的生成,從而促進血管通透性增加腫瘤的進展與轉移。腫瘤患者中高濃度的VEGF往往有更差的預后[7]。貝伐單抗是VEGF的單克隆抗體,可阻斷VEGF的生物學的作用,并可透過血腦屏障,從而控制腫瘤,同時減輕顱內水腫作用改善患者腦轉移癥狀,延長生存時間[8-12]。本研究試從分析貝伐珠單抗治療放療后腦轉移瘤進展或者復發患者療效。
1 資料與方法
1.1一般資料 2012年1月~2016年6月經過全腦放療后腦轉移瘤再次進展患者21例,其中肺癌腦轉移6 例、胃癌腦轉移5例、腦膠質瘤4例、乳腺癌腦轉移3例、結腸癌腦轉移2例、卵巢癌腦轉移1例。男12例、女9 例,年齡45~71歲(平均55.3歲)。臨床表現為顱內高壓、肌力減退、共濟失調以及認知功能障礙。腦轉移病灶有全腦放療治療史。接受放療平均生物劑量(40.4±5)Gy。
1.2診斷依據 所有腦轉移瘤患者原發病灶有病理支持。同時所有的腦轉移病灶符合以下影像學表現:CT或者MRI T1增強相表現為強化灶明確且水腫影明顯。從接受放療到腦轉移再次出現進展平均時間為6~22個月,平均時間9.2個月。
1.3方法 針對腦轉移瘤患者接受貝伐單抗5 mg/kg,每2周期重復1次,每2周期后第3周期治療前行MRI評價療效。癥狀嚴重者輔助予以甘露醇以及地塞米松控制水腫。
1.4觀察指標 每周期治療前記錄患者臨床癥狀及KPS評分,每2周期后第3周期前行MRI檢查,比較治療前后T1WI增強相病灶體積及水腫體積(水腫區體積-病灶區體積)把患者頭顱磁共振 T1WI增強相序列和 T2WI相序列分別通過網絡傳輸至TPS,分別勾畫每層增強病灶區或水腫區,通過UNICORN-3D 軟件計算體積。
1.5統計方法 采用 SPSS 16.0 軟件對治療前后腫瘤體積大小變化和水腫區體積變化行配對t檢驗,P<0.05為差異有統計學意義。
2 結果
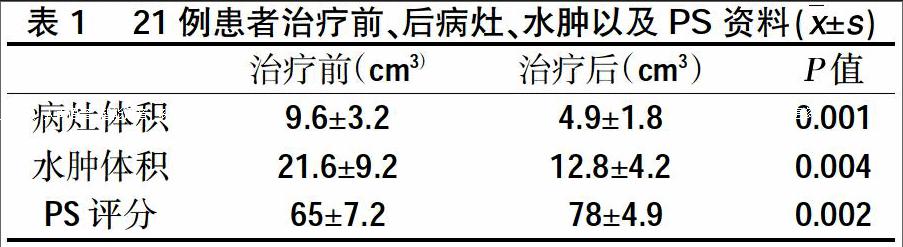
2.1腦轉移瘤病灶MRI T1WI增強相變化 所有患者均完成至少4個周期貝伐朱單抗維持治療。第5周期前(即貝伐珠單抗使用第9 w)行頭顱MRI評價。20例患者的頭顱病灶及水腫均有不同程度的減小,治療之前頭顱病灶體積(9.6±3.2)cm3,治療4周期后體積為(4.9±1.8)cm3。治療前后體積行t檢驗有統計學差異(P=0.001)。腫瘤病灶周邊水腫MRI T2WI變化:貝伐珠單抗治療第3周期前(即貝伐珠單抗使用第9 w)行頭顱MRI評價。20例患者的頭顱病灶周邊水腫均有不同程度的減小,治療之前頭顱病灶體積(21.6±9.2)cm3,治療后體積為(12.8±4.2)cm3。治療前后體積行t檢驗有統計學差異。(p=0.004)。治療前患者KPS評分(65±7.2), 4周期治療后第5周期治療前KPS評分(78±4.9)(P=0.002)。
2.2經過2個周期貝伐珠單抗治療后患者臨床癥狀(頭暈,惡心、共濟失調)較前均有不同程度的明顯改善。
2.3不良反應 20例患者行貝伐珠單抗治療中均未出現腦出血。其中有1例出現2級高血壓,經予以貝那普利對癥治療后控制癥狀。1例出現鼻出血,出血量約5 ml。
3 討論
貝伐珠單抗是一種單克隆抗體,主要抑制血管內皮生長因子,主要用于轉移性癌癥[8]。在1997年,1例29歲女性肝癌腦轉移患者在接受貝伐珠單抗I期臨床實驗過程中出現致命性的腦出血,之后貝伐珠單抗一直被排除應用在腦轉移瘤患者[13]。但FDA并無此禁忌,部分患者為腦轉移癌進展期,是否可用貝伐珠單抗值得探討。II期PASSPORT研究顯示入組腦轉移治療無進展或出血的非鱗癌患者,二線治療給予貝伐珠單抗聯合化療或厄洛替尼,可評價的106例患者中,未見到2度以上腦出血。結果顯示貝伐珠單抗治療腦轉移瘤是安全可行的[14]。之后研究發現在肺癌腦轉移患者中,接受貝伐珠單抗治療與否患者腦出血的發生幾率無明顯差異[15]。Levy采用全腦放療聯合貝伐珠單抗治療腦轉移瘤發現貝伐珠單抗的加入并沒有增加腦轉移瘤出血的風險[16]。Ranpura和中國學者在發現薈萃分析中發現貝伐珠單抗可增加心肌缺血的發作,但較對照組中并未增加腦出血發生[17-18]。貝伐單抗是一種重組人源化抗VEGF單克隆抗體,可抑制內皮細胞增殖和新生血管形成,使異常的血管內皮正常。從而阻止腫瘤血管生成和腫瘤生長,同時具有減少滲出,減輕顱內高壓作用[19-20]。而不良毒副反應較化療藥物明顯減輕,現已經廣泛應用在結直腸癌、非小細胞肺癌、腦膠質瘤等惡性腫瘤的治療。血管的生成是腫瘤惡化的特征之一,腫瘤的惡性程度越高,組織中的VEGF的表達也越高,常提示預后不良[21-22]。
本研究采用經過放療后腦轉移瘤進展患者,經過貝伐珠單抗應用后患者顱腦腫瘤較前均有明顯減小,且瘤周水腫較治療前均有明顯緩解。患者的生活質量,中位生存時間和1年生存率較目前常規的治療有明顯改善。
綜上所述,針對放療后腦轉移瘤復發或者轉移患者貝伐珠單抗可以有效控制腦轉移瘤的發展,減輕顱內水腫。明顯改善腦轉移瘤患者腦占位的癥狀以及生活質量,在治療過程中并未出現明顯的致命腦出血等副反應。因此貝伐珠單抗應用腦轉移瘤患者臨床療效較好,毒副反應較輕。可能是一種較為理想且有效的治療手段。
參考文獻:
[1]Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology[J].J Neurooncol,2005,75(1): 5-14.
[2]Tsao MN, Lloyd N, Wong R et al. Whole brain radiotherapy for the treatment of multiple brain metastases[J].Cochrane Database Syst Rev ,2006,4(3):99-99.
[3]Tsao MN, Lloyd N, Wong RK et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases[J].Cochrane Database Syst Rev,2012,(4): CD003869.
[4] Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy[J]. J Clin Oncol,2002,20(21): 4368-4380.
[5] LS Kim,S Huang, W Lu,et al Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice[J].Clin Exp Metastasis,2004,21(2):107-118.
[6]Jain RK, di Tomaso E, Duda DG et al. Angiogenesis in brain tumours[J].Nat Rev Neurosci,2007, 8(8): 610-622.
[7]Ebbers HC, van Meer PJ, Moors EH, et al. Measures of biosimilarity in monoclonal antibodies in oncology: the case of bevacizumab[J].Drug Discov Today,2013,18(17-18): 872-879.
[8]Besse B, Lasserre SF, Compton P, et al. Bevacizumab safety in patients with central nervous system metastases[J].Clin Cancer Res,2010, 16: 269-278.
[9]Lois A. Lampson.Monoclonal antibodies in neuro-oncology Getting past the blood-brain barrier[J].2011,3(2):153-160.
[10] Y Yoshida,S Hoshino N Aisu,et al.Efficacy of XELOX plus Bevacizumab in Brain Metastasis from Rectal Cancer[J].Case Report in Oncology,2014,7(7):117-121.
[11]Ferrara N, Gerber HP, LeCouter,et al.The biology of VEGF and its receptors[J].Nat Med,2003,9(6):669-676.
[12] Ay I, Francis JW, Brown RH. et al.VEGF increases blood-brain barrier permeability to Evans blue dye and tetanus toxin fragment C but not adeno-associated virus in ALS mice[J].Brain Res,2008,1234(3):198-205.
[13]Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer[J].J Clin Oncol,2001,19(3): 843-850.
[14]Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases[J].J Clin Oncol, 2009,27(31): 5255- 5261.
[15]Craig P, Carden, James M.G. Larkin, et al. What is the risk of intracranial bleeding during anti-VEGF therapy[J].Neuro-oncology,2008,10(4):624-630.
[16]C Levy, D Allouache,J Lacroix,et al. REBECA: a phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours[J].2014,25(12):2351-2356.
[17]Ranpura V, Hapani S, Chuang J, et al. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials[J].2010,49(3): 287-297.
[18]Pei-Yuan Zuo., Xing-Lin Chen, Yu-Wei Liu,et al. Increased Risk of Cerebrovascular Events in Patients with Cancer Treated with Bevacizumab: A Meta-Analysis[J].Plos One,2014,9(7):e102484.
[19]Ignoffo RJ. Overview of bevacizumab: a new cancer therapeutic strategy targeting vascular endothelial growth factor[J].Am J Health Syst Pharm, 2004, 61(21 Suppl 5): S21-26.
[20] Wang Y, Fei D, Vanderlaan M, et al. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro[J].Angiogenesis, 2004, 7(4): 335-345.
[21]Fujisawa T, Watanabe J, Akaboshi M, et al. Immunohistochemical study on VEGF expression in endometrial carcinoma--comparison with p53 expression, angiogenesis, and tumor histologic grade[J].J Cancer Res Clin Oncol, 2001, 127(11): 668-674.
[22]Ebbers HC, van Meer PJ, Moors EH, et al. Measures of biosimilarity in monoclonal antibodies in oncology: the case of bevacizumab[J].Drug Discov Today, 2013,18(17-18): 872-879.編輯/金昊天

