以脈沖萃取柱利用三異辛胺從高濃度鈾溶液中回收鈾
Mohamed Fathi El-ShahatHisham Mohamed KamalReda Abd El-Gawad GhazallaWalid Mohamed Morsy*,(Faculty of Science,Inorganic Chemistry Department,Ain Shams University,Cairo,Egypt) (Nuclear Materials Authority,Cairo,Egypt)
以脈沖萃取柱利用三異辛胺從高濃度鈾溶液中回收鈾
Mohamed Fathi El-Shahat1Hisham Mohamed Kamal2Reda Abd El-Gawad Ghazalla2Walid Mohamed Morsy*,2
(1Faculty of Science,Inorganic Chemistry Department,Ain Shams University,Cairo,Egypt) (2Nuclear Materials Authority,Cairo,Egypt)
利用三異辛胺(TOA)純化Gattar小型試驗工廠的高濃度鈾溶液(洗脫液的鈾濃度7 g·L-1),研究了脈沖萃取柱的性能。利用實驗室級脈沖萃取柱進行了實驗室規模的溶劑萃取實驗和后續實驗。結果表明,在室溫、pH=1和有機相與水相的比例(VO/VA)約為1.8∶1時,加入二(2-乙基己基)磷酸(D2EHPA)使其與TOA的比例(VD2EHPA/VTOA)為2∶3,可使萃取克服Cl-的抑制效應,提高效率。將結論用于考察試驗工廠級萃取柱的流體力學和傳質性能,結果表明用脈沖萃取柱萃取鈾可以達到97%的萃取效率,具有可行性。
洗脫液;氯;溶劑萃取法;脈沖萃取柱
0 Introduction
The eluate solution was produced by purification /concentration of leach liquor which is derived from heap leaching of uranium Gattar crushed ore using diluted sulfuric acid solution and an anion exchangeresin.However,thedirecturanium precipitation from this eluate was indeed low grade besidesincludingseveralkindsofimpurities. Therefore,the present work was so formulated to upgrade the Gattar mini pilot plant eluate via a solvent extraction procedure,i.e.an Eluex step prior to uranium precipitation.The presence of chloride in sulfate aqueous systems has a negative impact on the solvent extraction process for uranium recovery using tertiary amine reagents[1-2].When large amount of chloride presents in the solution, not only uranium loading is reduced significantly due to the competing extraction,but also the selectivity for uranium over Fe(Ⅱ)is reduced due to the extraction of FeCl4[3-5].

To realize this objective,it was found greatly advantageous to apply the pulsed column technique in the suggested Eluex procedure[6].Traditionally, pulsed columns were designed based on simple plug flow behavior,which does not incorporate the non-ideal flow effects caused by axial mixing.The mass transfer between the flowing liquid phases in an extraction column depends on the physical properties of phases and especially contact interfacial area between them.The interfacial area available for mass transfer in a counter-current extraction tower depends upon the volume fraction or holdup which defined as the fraction of the active column section volume occupied by the dispersed phase,as well as on the mean droplet size.
In addition,a set of correlations have been developed for standard internals to predict maxflux(Vmax)and pulsation intensity(FA)at max-flux and,for a given phase ratio and with the desired phase continuity-organic or aqueous.Max-flux was selected and correlated using Equation 1:

Pulsation intensity from the mixer-settler(MS)to the dispersion(D)is calculated by[7]:

Wheredispersedphaseholdupwascorrelated directly from the physical data,energy input and flow rates.The fit of their work was quite good (average deviation of 13%)[8]:

1 Experimental
The batch experiments of uranium extraction were carried out in beakers under mechanical agitation at room temperature and a ratio of organic phase and aqueous phase VO/VA)equal to 1.Nine synthetic solutions with different Cl-ion concentration(20~100 g·L-1)but with same other elements concentration were prepared.The experiments were controlled by sampling and analyzing the effluent flows from both aqueous and organic phases then uranium extraction efficiency will be calculated after phase separation,by analysis of uranium volumetrically in the aqueous phase and in organic ones by calculating the difference.
Analytical grade tri-octyl amine(TOA)and di (2-ethylhexyl)phosphoric acid(D2EHPA)from BDH Chemicals(assay 99.5%)was used in Egyptian pure kerosene.Physical properties of concentrated uraniumsolutionandorganicsolventwere measured by the Petroleum Inst.Res.of Egypt.
The continuous extraction experiments were carried out in a countercurrent system using in apulsed perforated-plate extraction column of 120 mm long with diameter of 50 mm,enclosing a stack of sieve plates.Below the plate section was a 120mm expanded glass section enclosing a PVC solventdistributorsupportedonapiston-type pulsing unit,which imparted a sinusoidal motion to the fluids of the column.Totally,20 PVC sieve plates were arranged alternately and spaced 50mm apart in the column.They had a 2 m perforation diameter and 22.7%free area,where the flow rates of the two phases were indicated by two rotameters as illustrated in Fig.1.Before carrying out the experiments,both phases were mutually saturated, andthepulseamplitudeandfrequencywere adjusted to the desired values.The continuousphase and the dispersed-phase flow rates were then set to the required flow rate,and the system was stabilized to allow steady state to be reached.Then, the inlet and outlet flows were stopped simultaneously.The dispersion was then allowed to coalesce at the interface.The holdup was then measured either by determining the change of interfacial height or by displacing the solvent layer into a measuring cylinder.

Fig.1Sketch of the pulsed perforated plate column
2 Results and discussion
2.1Batch experiment
As shown in Table 1,the main problems derived during purification were the presence of chloride and iron in high concentration 32,1.25 g· L-1).As the chloride concentration increased to 8 g·L-1,the loaded uranium concentration in the organic solution reduced to half compared with no chloride presence in the feed solution[9].
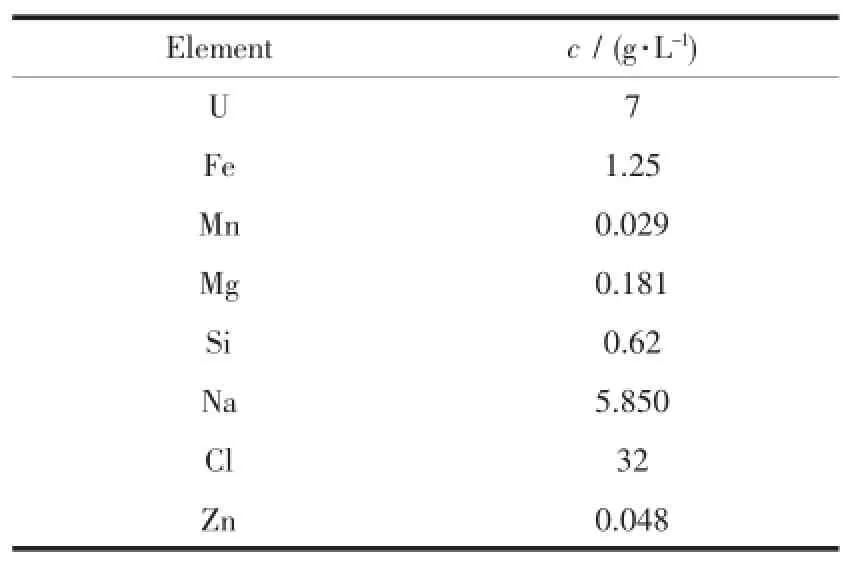
Table 1Chemical composition of the studied eluate
2.1.1Determination of contact time and pH value
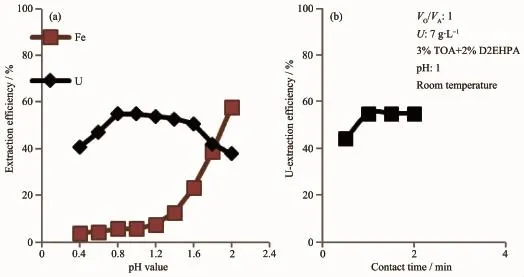
Fig.2Effect of pH value(a)and contact time(b)on uranium extraction from eluate by 3% (volume fraction,the same below)TOA+2%DEHPA
The extraction of uranium was examined at different pH values.As shown in Fig.2,U(Ⅱ)extraction increases with increasing acidity and becomes quantitative at pH 1,and the uranium uptake percent increases with increasing contact time till equilibrium after 1 min.
2.1.2Determination of optimum uranium and chloride concentration
Extraction studies shows that the uranium concentration in the organic phase has remained constant at 3.85 g·L-1when the input concentration attained wasnt higher than 5 g·L-1and only increased to 3.9 g·L-1when the latter increased to 8 g·L-1.The obtained results in Fig.3 have revealed that 60 g·L-1chloride can be used as an upper limit of undergoing extraction by the operating mixed Amex solvent and the eluted solution should be recycled to lower the chloride content and exceeding the uranium concentration.
2.1.3Determination of mixed solvent composition
It is obvious that the absence of D2EHPA in the working 3%TOA solvent has not been able to extract uranium above 0.6%while it is extractable to 90.4%of iron from the working eluate.This result is most probably attributed to excessive Clextraction by TOA instead of uranyl sulfate in addition to iron extraction as FeCl4.Increasing the D2EHPA concentration thereafter from 0.5%to 2% has decreased the iron extraction from 38.7%to 5.7%respectively;however,at 2.5%D2EHPA,it re-increased to 21.6%.

Fig.3Effect of uranium(a)and chloride(b)concentration on uranium extraction from eluate by 3%TOA+2%D2EHPA
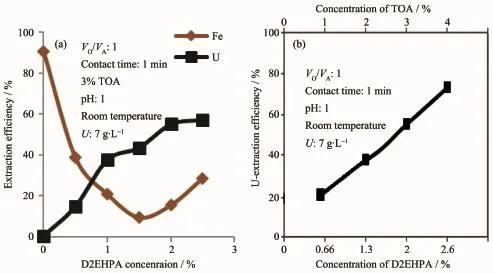
Fig.4(a)Effect of D2EHPA concentration on uranium and iron extraction from eluate by 3%TOA; (b)Effect of TOA-D2EHPA concentration on uranium extraction
It′s quite obvious that both the uranium extraction efficiency and its distribution coefficient increasewiththeincreaseofmixedsolventconcentrations at VTOA/VD2EHPAof 3∶2.The synergistic mixture of 3%TOA plus 2%D2EHPA in kerosene was chosen as the optimum concentrations for acceptable uranium saturation capacity (3.85 g·L-1)and which approaches the theoretical capacity of the working TOA percent.This is due to that as the solvent concentration increases,the interfacial areas between the two phases decrease as well as the settling time.
2.1.4Effect of VO/VAand the McCabe-Thiele extraction diagram from eluate
Applying the previouslyobtainedoptimum conditions with VO/VAalteration shows that the uranium extraction efficiency has increased from 55.0%up to 96.4%as the applied VO/VAincreased from 1∶1 to 2∶1,which confirm actually that the working solvent would be saturated with cUof 3.85 g·L-1.To construct the corresponding McCabe-Thiele extraction diagram,a proper operating line was fitted to the equilibrium isotherm curve,whose slope(VO/VA)was found to be about 1.8.

Fig.5McCabe-Thiele extraction diagram of uranium from eluate by 3%TOA+2%D2EHPA mixed solvent
2.2Continuous experiment
2.2.1Physical and theoretical aspects of operated solutions
Physicalpropertiesofthesolutionsplay important role in the extraction process to predict maximum flux and pulsation intensity at maximum flux and holdup,so that measured and tabulated in following tables. Table 3 shows that the system was characterized by high interfacial tension which allows large area for extraction and need a wide range of frequency and amplitude.The choice of pulsation frequency which can be attributed to the solvent type,low pulse velocities(high frequency and low amplitude)are required to avoid emulsification[10-11].
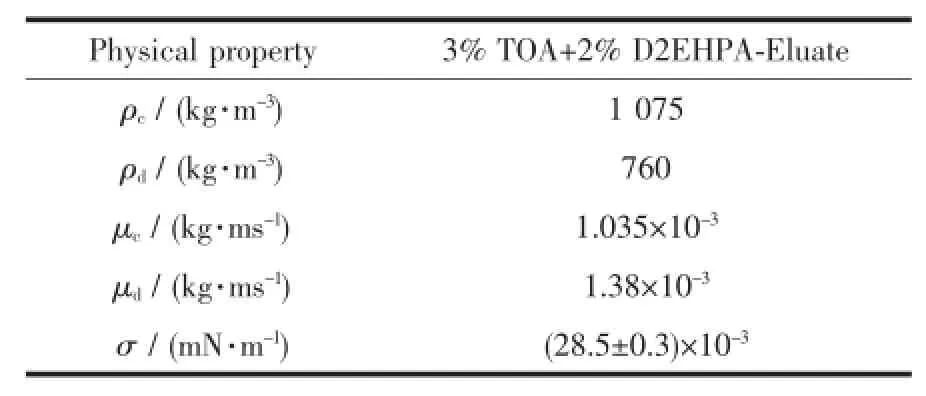
Table 2Physical properties of operated liquid system
2.2.2Optimizing parameters of a sieve plate pulsed column for uranium extraction
Calculated parameters in Table 4 would be used as guidelines to determine the range of pulsation intensity,frequency and flux for 3% TOA+2%D2EHPA-eluate system under previous obtained VO/VA.
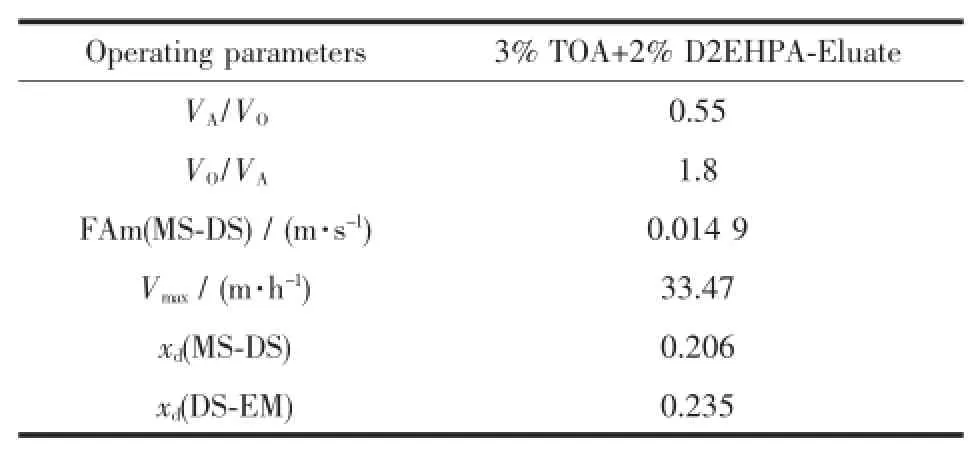
Table 3Calculated theoretical operating parameters of extraction process in pulsed column

Fig.6Effect of pulsation intensity and flux on uranium mass transfer coefficient in pulsed column

Fig.7Effect of Pulsation intensity and Flux on holdup during extraction in pulsed column
The obtained results show that as the pulsation intensity increase from 0.018(dispersion/emulsion regime boundary)to 0.026 m·s-1the uranium extraction coefficient increased from 0.469 to 5.7 in operated systems.After that the increase in pulsation intensity from 0.026 to 0.032 m·s-1will lead to decrease in mass transfer to 2.07 which owing to start of flooding phenomenon.In the same time the holdup fraction increases periodically from 0.128 to 0.398 as pulsation intensity increases from 0.026 to 0.032 m·s-1which tend to be applicable with calculated one.However,when the uranium extraction coefficient was increased from 5.17 to 8.7,the flux increases from 30 to 32.Then it was decreased to 5.7 with the flux increase from 32 to 38 m·h-1.While the increase in pulsation frequency resulted in substantial decrease in the time required to attain steady state for percentage of uranium in extract phase by increasing the flux from 30 to 32 m·h-1.The experimental measured holdupat different values of operating conditions shows good performance of the constructed pulsed column since it′s nearly applicable with calculated one.
3 Conclusions
Purification and extraction of uranium from Gattar mini pilot plant eluate solution with mixed Amex solvent(TOA+D2EHPA)was investigated. The tests performed at lab scale and continuous level using pulsed column allowed to a better understanding of the hydrodynamics of the pulsed column.The feasibility of operating the uranium purification of Gattar mini pilot plant eluate in a pulsed column was thus demonstrated and successes in determining the best extraction regimes and the operating parameters at which the flooding start.
Nomenclature:
Vc,Vd/(m·h-1):continuous and dispersed flow per unit of time and cross section area
FA/(m·s-1):multiply pulse amplitude by frequency(number of pulses)
σ/(mN·m-1):interfacial tension
α:free fractional plate area
xd:dispersed phase holdup fraction
h/m:compartment height
d/m:inner diameter of the column
d0/m:plate hole diameter
μc:continuous phase viscosity
μd:dispersed phase viscosity
hs:center-to-center plate spacing,standard plate spacing=0.05 m
ρ:density
ρ*:density of water at 20℃,998 kg·m-3
Δρ:density difference between phases
DU:uranium extraction coefficient,DU=cU(orgphase)
k1,k2:varies from 1.1×106,50.26 for masstransfer from the dispersed to continuous phase and 2.14×106,44.53 for the continuous to the dispersed one respectively
References:
[1]Yakubu N A,Dudeney W L.Hydrometallurgy,1987,18(1): 93-104
[2]Girgin S,Acarkan N,Ali S A.J.Radioanal.Nucl.Chem., 2002,251(2):263-271
[3]Ivanova I,Fraser K S,Thomas K G,et al.ALTA Uranium Conference.Perth,Australia,2009.
[4]Soldenhoff K,Wilkins D,Shamieh M,et al.Proceedings of the International Symposium on the Process Metallurgy of Uranium.Quebec,Canada,2000:339-351
[5]Zhu Z W,Cheng C Y.Parker Centre/CSIRO Process Science and Engineering.WA,Australia,2010:6152
[6]Soldenhoff K,Wilkins D,Shamieh M,et al.ISEC 2005, Solvent Extraction for Sustainable Development.Beijing:[s.n.], 2005:214-219
[7]Lorenz M,Haverland H,Vogelpohl A.Chem.Eng.Technol., 1990,13:411-422
[8]Barbara E,Michael R,James F R,et al.Ullmann′s Encyclopedia of Industrial Chemistry:Vol.B3,Unit Operations II, Weinheim,Basel,Cambridge,New York:Wiley&Sons,1988: 6-26
[9]Jahya A B,Stevens G W,Pratt H R C.Proceedings of the Chemecá,27th Australian Chemical Engineering Conference. Newcastle,Australia,1999.
[10]Godfrey J C,Houlton D A,Marley S T,et al.Chem.Eng. Res.Des.,1988,66:446-457
[11]Kumar A,Hartland S.Ind.Eng.Chem.Res.,1989,28:1507-1513
Recovery of Uranium from Its Concentrated Solution by Tri-octyl Amine Using Pulsed Column
Mohamed Fathi El-Shahat1Hisham Mohamed Kamal2Reda Abd El-Gawad Ghazalla2Walid Mohamed Morsy*,2
(1Faculty of Science,Inorganic Chemistry Department,Ain Shams University,Cairo,Egypt) (2Nuclear Materials Authority,Cairo,Egypt)
The performance of pulsed column for purification of concentrated uranium liquor(cU=7g·L-1in eluate) produced in Gattar mini pilot plant using tri-octyl amine(TOA)has been investigated.Bench scale studies on solvent extraction have been carried out followed by continues experiments using a laboratory pulsed column.It was found that the addition of di(2-ethylhexyl)phosphoric acid(D2EHPA)to TOA with the ratio(VD2EHPA/VTOA)at 2∶3 make it overcome the suppression effect of the Cl-ion and improve the reaction at possible ratio of organic phase and aqueous phase(VO/VA)of 1.8∶1,pH=1 under room temperature.The obtained bench scale results were chosen to test the hydrodynamic and mass transfer performance of the extraction column.Results of the laboratory plant generally demonstrated the feasibility of operating the extraction of uranium using pulsed column with 97%extraction efficiency.
eluate;chloride;solvent extraction;pulsed column
O614.62
A
1001-4861(2016)08-1427-07
10.11862/CJIC.2016.176
2015-12-12。收修改稿日期:2016-05-26。
*通信聯系人。E-mail:wmmorsy@yahoo.com

