三個(gè)包含酰胺型配體銅/鋅配合物的合成、結(jié)構(gòu)及熒光性質(zhì)
毛盼東 閆玲玲 吳偉娜*, 姚必鑫 劉珉琦 王元(河南理工大學(xué)化學(xué)化工學(xué)院,焦作454000)(河南理工大學(xué)物理與電子信息學(xué)院,焦作454000)(河南理工大學(xué)材料科學(xué)與工程學(xué)院,焦作454000)
三個(gè)包含酰胺型配體銅/鋅配合物的合成、結(jié)構(gòu)及熒光性質(zhì)
毛盼東1閆玲玲*,2吳偉娜*,1姚必鑫3劉珉琦3王元1
(1河南理工大學(xué)化學(xué)化工學(xué)院,焦作454000)
(2河南理工大學(xué)物理與電子信息學(xué)院,焦作454000)
(3河南理工大學(xué)材料科學(xué)與工程學(xué)院,焦作454000)
合成并通過(guò)單晶衍射表征了3個(gè)配合物[CuLCl2]·CH3CN(1),[CuLBr2]·CH3CN(2)和[ZnL(NO3)2]·CH3CN(3)(L=2-(5-氯-8-喹啉氧基)-1-(吡咯烷-1-基)乙酮)。在配合物1和2中,五配位的銅離子采取扭曲的四方錐配位構(gòu)型,與來(lái)自配體L的2個(gè)氧原子和1個(gè)氮原子及2個(gè)鹵離子配位。而在配合物3中,鋅離子與1個(gè)三齒配位的配體L,1個(gè)單齒配位的硝酸根和1個(gè)雙齒配位的硝酸根配位,配位構(gòu)型為扭曲的八面體。乙腈溶液中,配合物1和2在410 nm處的最大熒光發(fā)射峰與配體L的相似,強(qiáng)度有所降低。而配合物3由于配體到鋅離子之間的能量轉(zhuǎn)移,最大熒光發(fā)射峰紅移至430 nm。
銅配合物;鋅配合物;酰胺型配體;晶體結(jié)構(gòu);熒光
It is well known that the amide type ligands, which are effective chelating agents for lanthanide(Ⅱ)ions and have terminal-group effects,will shield the encapsulated ions from interaction with the surroundings effectively to achieve strong characteristic fluorescent emission of the centre metal ions[1-6].Besides, some acetamide ligands bearing quinolinyloxy unit have also been used for the recognition of important transition metal ions,such as Zn(Ⅱ),Cd(Ⅱ)or Hg(Ⅱ), due to the metal-induced fluorescence emission enhancement[7-8].Our previous work shows that this kind of ligands could coordinate to Cu(Ⅱ)/Zn(Ⅱ)ions to form stable complexes[9-10].Thus,in this paper,three Cu(Ⅱ)/ Zn(Ⅱ)complexes containing an amide type ligand were synthesized and characterized by X-ray diffraction.In addition,the fluorescence properties of all compounds are investigated in detail.
1 Experimental
1.1Materials and measurements
Solvents and starting materials for synthesis were purchasedcommerciallyandusedasreceived. Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.1H NMR spectra of the ligand L was acquired with Bruker AV400 NMR instrument in DMSO-d6solution with TMS as internal standard.The UV spectra were recorded on a PurkinjeGeneralTU-1800spectrophotometer. Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer.
1.2Preparations of the complexes
The ligand L(Scheme 1)was prepared according to literature methods[5],while using 2-chloro-1-(pyrrolidin -1-yl)ethanone instead of 2-chloro-N-phenylacetamide. Yield:69%;m.p.89~92℃.Elemental analysis for C17H13N2O2Cl(%):Calcd:C:65.29;H:4.19;N:8.96; Found:C:65.18;H:4.33;N:8.88.1H NMR(400 MHz,CDCl3):δ 8.97~8.98(1H,dd,Ar-H),8.50~8.53 (1H,dd,Ar-H),7.72~7.75(1H,q,Ar-H),7.67~7.69 (1H,d,Ar-H),7.13~7.17(1H,d,Ar-H),4.99(2H,s, CH2),3.53~3.56(2H,t,CH2),3.33~3.37(2H,t,CH2), 1.87~1.92(2H,m,CH2),1.77~1.82(2H,m,CH2).FTIR(cm-1):ν(C=O)1682,ν(C=N)1598,ν(Ar-O-C)1254.
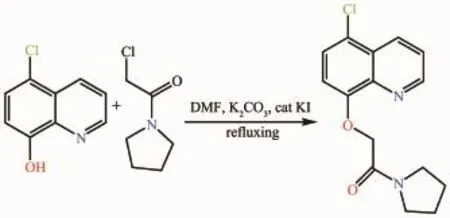
Scheme 1Synthetic scheme for ligand L
The ligand L(0.1 mmol)and CuCl2·2H2O(0.1 mmol)were dissolved in an acetonitrile solution(4 mL). After stirred for about 1 h,the mixture was filtered and set aside to crystallize at room temperature.The crystals suitable for single crystal X-ray analyses are obtained after few days.The synthesis of 2 and 3 is similar to that of 1,while using CuBr2and Zn(NO3)2· 6H2O instead of CuCl2·2H2O,respectively.
1:orange rods.Yield:59%(based on L).Anal. Calcd.for C17H18N3O2Cl3Cu(%):C,43.79;H,3.89;N, 9.01.Found(%):C,43.62;H,4.01;N,8.98.IR(KBr, cm-1):ν(C≡N),2 249,ν(C=O)1 624,ν(C=N)1 588, ν(Ar-O-C)1 242.
2:orange rods.Yield 68%(based on L).Anal. Calcd.for C17H18N3O2ClBr2Cu(%):C,36.78;H,3.27; N,7.57.Found(%):C,36.67;H,3.19;N,7.52.IR (KBr,cm-1):ν(C≡N),2 248,ν(C=O)1 622,ν(C=N) 1 589,ν(Ar-O-C)1 238.
3:colorless rods.Yield 68%(based on L).Anal. Calcd.for C17H18N5O8ClZn(%):C,39.18;H,3.48;N, 13.44.Found(%):C,39.07;H,3.39;N,13.52.IR (KBr,cm-1):ν(C≡N),2 247,ν(C=O)1 634,ν(C=N) 1 588,ν(Ar-O-C)1 244,ν1(NO3)1 485,ν4(NO3)1 385 and 1 302.
1.3X-ray crystallography
The X-ray diffraction measurement for complexes 1~3 were performed on a Bruker SMART APEXⅡCCDdiffractometerequippedwithagraphite monochromatized Mo Kα radiation(λ=0.071 073 nm) by using φ-ω scan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[11].The structures were solved bydirect methods and refined by fullmatrixleast-square on F2using the SHELX-97 program[12].All non-hydrogen atoms were refined anisotropically.The H atoms for water molecules are located from difference Fourier map and refined with restraints in bond length and thermal parameters.All the other H atoms were positioned geometrically and refined using a riding model.Detailsofthecrystalparameters,data collection and refinements for complexes 1~3 are summarized in Table 1.
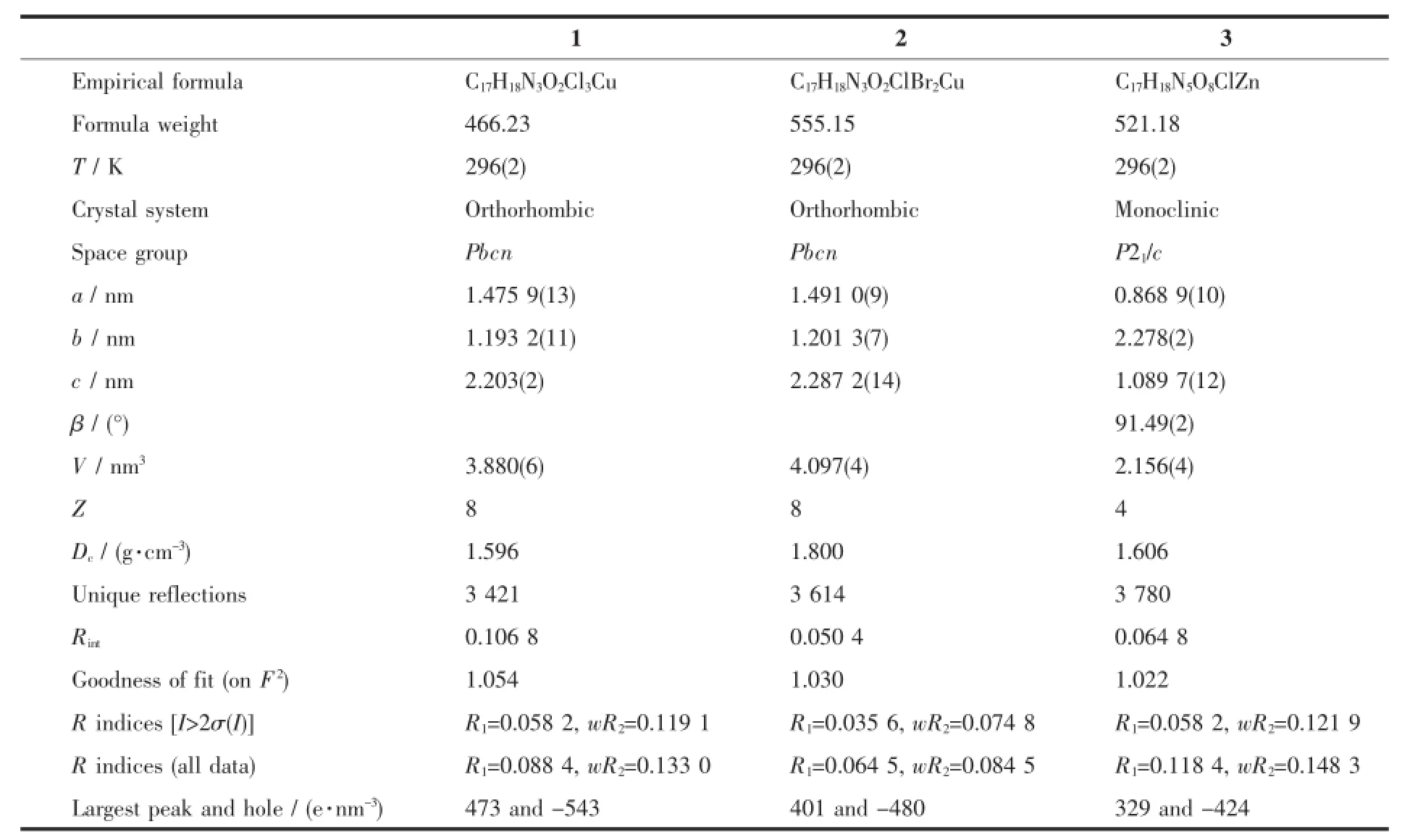
Table 1Selected crystallographic data for complexes 1~3
CCDC:1474712,1;1474713,2;1474714,3.
2 Results and discussion
2.1Crystal structures of the complexes
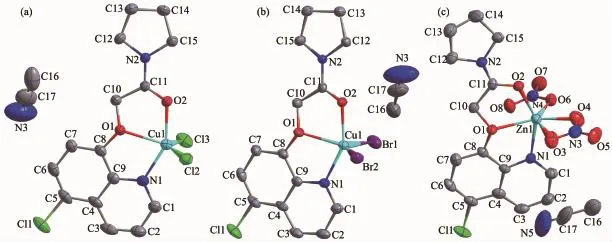
Fig.1Molecular structures of complexes 1~3(a~c)shown with 30%probability displacement ellipsoids

Table 2Selected bond lengths(nm)and angles(°)in complexes 1~3
Complexes1and2areisostructuraland crystallize in the orthorhombic,space group Pbcn.As shown in Fig.1,in each complex,the Cu(Ⅱ)ion is fivecoordinated by one amide ligand with NO2donor set and two halide anions(chloride for 1 and bromide for 2).Selected bond lengths and angles are summarized in Table 2.According to the Addison rule[9],the geometric index τ is 0.285 5 and 0.396 1 for Cu(Ⅱ) ion in complexes 1 and 2,respectively,indicating that the coordination geometry of Cu(Ⅱ)ion in each complex is a distorted tetragonal pyramid.It is worth noting that the coordination behavior of the ligand L in both complexes is quite different to those in[CuL12Cl2]·DMF and[CuL22Cl2],(L1=N-(4-chlorophenyl)-2-(quinolin-8-yloxy)acetamide,L2=N,N-diphenyl-2-(quinolin-8-yloxy) acetamide)[10].In the literature complexes,the carbonyl oxygen atom of the amide type ligands did not take part in coordination to center Cu(Ⅱ)ion.
By contrast,the Zn(Ⅱ)ion in complex 3(Fig.1c) is surrounded by one tridentate ligand L and two nitrate anions,one of which is monodentate and the other is bidentate,thus giving distorted octahedral coordination geometry.Its structure is different from that of[ZnL3(NO3)2]·CH3CN(L3=N-(naphthalen-1-yl)-2-(quinolin-8-yloxy)acetamide),in which the ligand L3is also tridentate while two nitrate anions are both monodentate[9].As expected,there are no classic hydrogen bonds in the structures of all three complexes.
2.2IR spectra
The IR spectra of free ligand L show strong band at 1 682 cm-1,which is attributable to stretch vibrations of the carbonyl group(ν(C=O)).The peak at 1 598 cm-1should be assigned to the ν(C=N),and the peak at 1 254 cm-1to ν(Ar-O-C),respectively.In three complexes,the ν(C=O),ν(C=N-N)and ν(quinoline C= N)shifts to lower wavenumber in three complexes, indicating that carbonyl oxygen,ethereal oxygen and quinoline nitrogen atoms take part in coordination[9]. In addition,the intense absorption bands in the spectra of complex 3 associated with the asymmetric stretching appear at 1 385,1 302 cm-1(ν4)and 1 485 cm-1(ν1),clearly establishing that two NO3-groups are monodentate and bidentate ligands,respectively[13]. The ν(C≡N)bands in complexes 1~3 appear at around 2 250 cm-1,clearly showing the existence of CH3CN molecules.It is in accordance with the resultof the crystal structure study.
2.3UV spectra
The UV spectra of L,and complexes 1~3 in CH3CN solution(concentration:1×10-5mol·L-1)were measured at room temperature(Fig.2).The spectra of L features two main bands located at 244(ε=156 000 L·mol-1·cm-1)and 316 nm(ε=19 500 L·mol-1·cm-1), which should be assigned to characteristic π-π* transitionscenteredonquinolineringandthe acetamide unit,respectively[9-10].Similar bands at 243 (ε=224 000 L·mol-1·cm-1)and 318(ε=42 900 L·mol-1·cm-1)nm,243(ε=198 000 L·mol-1·cm-1)and 322 (ε=40 000 L·mol-1·cm-1)nm,245(ε=90 000 L·mol-1·cm-1)and 319(ε=10 800 L·mol-1·cm-1)nm in complex 1~3,respectively.The spectra variations indicated that the ligand L takes part in the coordination in three complexes.
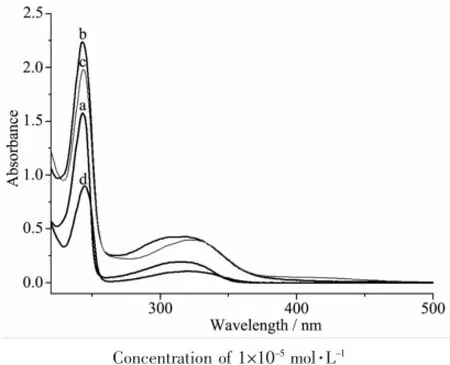
Fig.2UV spectra of L(a),1(b),2(c)and 3(d)in CH3CN solution at room temperature
2.5Fluorescence spectra
The fluorescence spectra of the ligand L and complexes 1~3 have been studied in CH3CN solution (concentration:1×10-5mol·L-1)at room temperature. Theresultsshowthattheemissionspectraof complexes 1 and 2 exhibit only one main peak at 410 nm when excited at 320 nm,which is similar as that of the ligand L(Fig.3).Compared with that of the ligand L,the fluorescence intensity of complexes 1 and 2 decreased dramatically,probably due to the inherent magnetic property of Cu2+ions[14].However, the emission band of complex 3 red-shifts to 430 nm (excited at 320 nm),indicating the energy transferring from the ligand L to the Zn(Ⅱ)ion[9,13].
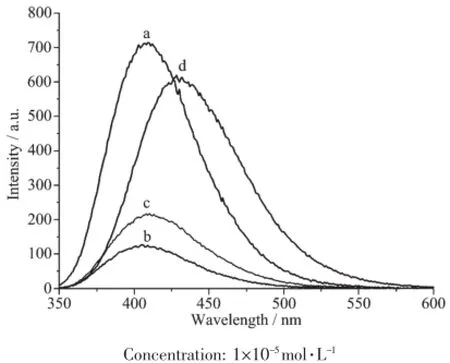
Fig.3Fluorescence emission spectra of L(a),1(b),2(c) and 3(d)in CH3CN solution at room temperature
References:
[1]Song X Q,Yu Y,Liu W S,et al.J.Solid State Chem.,2007, 180:2616-2624
[2]Song X Q,Wen X G,Liu W S,et al.J.Solid State Chem., 2010,183:1-9
[3]Sharma A K,Biswas S,Barman S K,et al.Inorg.Chim.Acta, 2010,363:2720-2727
[4]Munjal M,Kumar S,Sharma S K,et al.Inorg.Chim.Acta, 2011,377:144-154
[5]MAO Pan-Dong(毛盼東),CHEN Liang(陳亮),WU Wei-Na (吳偉娜),et al.Chinese J.Inorg.Chem.(無(wú)機(jī)化學(xué)學(xué)報(bào)), 2016,32:336-342
[6]Wu W N,Tang N,Yan L.J.Fluoresc.,2008,18:101-107
[7]Song K C,Kim J S,Park S M,et al.Org.Lett.,2006,8:3413 -3416
[8]Zhou X,Li P,Shi Z,et al.Inorg.Chem.,2012,51:9226-9231
[9]WU Wei-Na(吳偉娜),WANG Yuan(王元),TANG Ning(唐寧).Chinese J.Inorg.Chem.(無(wú)機(jī)化學(xué)學(xué)報(bào)),2012,28:425-428
[10]CAI Hong-Xin(蔡紅新),WU Wei-Na(吳偉娜),WANG Yuan (王元).Chinese J.Inorg.Chem.(無(wú)機(jī)化學(xué)學(xué)報(bào)),2013,29: 845-849
[11]Sheldrick G M.SADABS,University of G?ttingen,Germany, 1996.
[12]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of G?ttingen, Germany,1997.
[13]LI Xiao-Jing(李曉靜),WU Wei-Na(吳偉娜),XU Zhou-Qing (徐周慶),et al.Chinese J.Inorg.Chem.(無(wú)機(jī)化學(xué)學(xué)報(bào)), 2015,31:2256-2271
[14]Qin J C,Wang B D,Yang Z Y,et al.Sens.Actuators B, 2016,224:892-898
Syntheses,Crystal Structures and Fluorescence Properties of Three Cu(Ⅱ)/Zn(Ⅱ)Complexes with an Amide Type Ligand
MAO Pan-Dong1YAN Ling-Ling*,2WU Wei-Na*,1YAO Bi-Xin3LIU Min-Qi3WANG Yuan1
(1College of Chemistry and Chemical Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(2School of Physics and Electronic Information Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(3School of Materials Science and Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Three complexes,[CuLCl2]·CH3CN(1),[CuLBr2]·CH3CN(2)and[ZnL(NO3)2]·CH3CN(3)(L=2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethanone),were synthesized and characterized by X-ray diffraction.In each of complexes 1 and 2,the five-coordinated Cu(Ⅱ)ion is in a distorted tetragonal pyramid with a NO2donor set from one ligand L and two halide anions.However,the Zn(Ⅱ)ion in complex 3 is surrounded by one tridentate ligand L and two nitrate anions,one of which is monodentate and the other is bidentate,thus giving distorted octahedral coordination geometry.In CH3CN solution,complexes 1 and 2 exhibit similar peak at 410 nm as that of the ligand L,while with a decrease of the fluorescence intensity.However,the emission band of complex 3 red-shifts to 430 nm because of energy transferring from the ligand L to the Zn(Ⅱ)ion.CCDC:1474712,1; 1474713,2;1474714,3.
Cu(Ⅱ)complex;Zn(Ⅱ)complex;amide type ligand;crystal structure;fluorescence
O614.121;O614.24+1
A
1001-4861(2016)08-1476-05
10.11862/CJIC.2016.182
2016-04-27。收修改稿日期:2016-06-17。
國(guó)家自然科學(xué)基金(No.21001040)、河南省教育廳自然科學(xué)基金(No.12B150011,14B150029)資助項(xiàng)目。
*通信聯(lián)系人。E-mail:yll@hpu.edu.cn,wuwn08@hpu.edu.cn;會(huì)員登記號(hào):S06N6704M1112。
 無(wú)機(jī)化學(xué)學(xué)報(bào)2016年8期
無(wú)機(jī)化學(xué)學(xué)報(bào)2016年8期
- 無(wú)機(jī)化學(xué)學(xué)報(bào)的其它文章
- 4,4′-聯(lián)吡啶橋聯(lián)的三個(gè)銅有機(jī)膦酸配合物的合成、結(jié)構(gòu)及磁性
- 由芳香羧酸和4,4′-二(1-咪唑基)苯砜為配體構(gòu)筑的兩個(gè)配合物的合成、晶體結(jié)構(gòu)及熒光性質(zhì)
- 由4-甲基-1,2,3-噻二唑-5-甲酸構(gòu)筑的銅配合物的合成、晶體結(jié)構(gòu)及與DNA作用
- 以5-甲基-3-吡唑甲酸為配體的鈷(Ⅱ)、鎳(Ⅱ)配合物的合成、晶體結(jié)構(gòu)和性質(zhì)
- 特戊酸和含氮配體構(gòu)筑的Ln(Ⅱ)配合物的結(jié)構(gòu)、熱穩(wěn)定性和熒光性質(zhì)
- 聚多巴胺功能化的四氧化三鈷納米復(fù)合材料的制備及電催化性能
