由芳香羧酸和4,4′-二(1-咪唑基)苯砜為配體構筑的兩個配合物的合成、晶體結構及熒光性質
徐涵(黃山學院化學化工學院,黃山4504)(南京大學化學化工學院,人工微結構科學與技術協同創新中心,南京0093)
由芳香羧酸和4,4′-二(1-咪唑基)苯砜為配體構筑的兩個配合物的合成、晶體結構及熒光性質
徐涵1,2
(1黃山學院化學化工學院,黃山245041)
(2南京大學化學化工學院,人工微結構科學與技術協同創新中心,南京210093)
通過水熱法得到了2個配位聚合物{[Cd(L)(oba)]·3.5H2O}n(1)和{[Cd(L)2](p-bdc)}n(2)(L=4,4′-二(1-咪唑基)苯砜,H2oba=4,4′-聯苯醚二甲酸,p-H2bdc=對苯二甲酸),對它們進行了元素分析、紅外光譜分析,并利用X射線衍射測定了它們的單晶結構。晶體結構分析表明,配合物1具有四連接二重穿插的三維網絡結構,其拓撲為{65·8}。配合物2具有四連接的二維結構,其拓撲為{44· 62}。此外,在室溫下對2個配合物進行了熒光分析。
鎘(Ⅱ)配位聚合物;咪唑配體;晶體結構;熒光性質
Theconstructionof metal-organic framework (MOFs)hasattractedmuchattentionfortheir intriguing topologies as well as potentional application in gas and small molecule storage[1-2],catalysis[3-4],drug delivery[5]and so on.During the attainment of desirable frameworks,many factors can influence the construction progress,for instance,coordination tendency of metalcenters,ligand-to-metalratios,reactiontemperature,reagents concentration,an so on[6].It is well known that combining different ligands in a complex offers greater tunability of the structural framework than using a single ligand.Hence,a mixed-ligand is undoubtedly a good choice for the construction of new polymeric structures[7-8].Imidazole ligands have been used in the synthesis of remarkable MOFs which posses excellent coordination ability allowing free rotation of the imidazole ring to meet the requirement of coordination geometries of metal ions. Until now,a large number of ingenious MOFs have been synthesized and designed based on imidazole ligands[9-10].
Recently,we have successfully designed and synthesized a new V-shaped imidazole ligand 4,4′-bis (imidazol-l-yl)-phenyl sulphone(L),which may be regardedasemi-flexibleligand.Consideringthe mixedN-containgligandsandpolycarboxylate possesing more tunable factors in the construction of novel MOFs,we carried out the reactions of the ligand L together with different carboxylate ligands and cadmium nitrate.
Herein,we report the syntheses,crystal structures, and properties of two new coordination polymers {[Cd(L)(oba)]·3.5H2O}n(1)and{[Cd(L)2](p-bdc)}n(2), and the details of their syntheses,structures,and properties were investigated below.
1 Experimental
1.1Materials and measurement
All the chemicals except the ligand L were commercially purchased and used without further purification.The ligand L was synthesized according to literature method[11].Elemental analyses of C,H,N wereperformedonaElementarVarioMICRO Elemental Analyzer.Fourier transformed Infrared(FTIR)spectra were obtained on a Bruker Vector 22 FTIRspectrophotometerbyusingKBrpellets. Thermogravimetrical analyses(TGA)were performed on a Perkin-Elmer thermal analyzer under nitrogen with a heating rate of 10℃·min-1.Powder X-ray diffraction(PXRD)patterns were collected in the 2θ range of 5°~50°with a scan speed of 0.2°·s-1on a Bruker D8 Advance instrument using a CuKα radiation(λ=0.154 056 nm)at room temperature,in which the X-ray tube was operated at 40 kV and 40 mA.Luminescentspectrawererecordedwitha SHIMAZU VF-320 X-ray fluorescence spectrophotometer at room temperature(25℃).
1.2Synthesis of compounds
{[Cd(L)(oba)]·3.5H2O}n(1):A mixture of Cd(NO3)2·6H2O(30.8 mg,0.1 mmol),H2oba(25.8 mg,0.1 mmol) and L(37.2 mg,0.1 mmol)was dissolved in 5 mL of DMF/CH3CN/H2O(1∶2∶2,V∶V∶V).The final mixture was placed in a Parr Teflon-lined stainless steel vessel(15 mL)under autogenous pressure and heated at 105℃for two days.Colorless block crystals were obtained.The yield of the reaction was ca.53%based on L.Anal.Calcd.for C32H29CdN4O10.5S(%):C,49.10; H,3.71;N,7.16.Found(%):C,49.14;H,3.68;N, 7.20.IR(KBr,cm-1):3 403(s),3 139(s),1 606(m), 1 564(m),1 458(m),1 401(s),1 255(w),1 127(w), 1 046(m),964(w),819(w),780(w),735(m),564(w).
{[Cd(L)2](p-bdc)}n(2):A mixture of Cd(NO3)2· 6H2O(30.8 mg,0.1 mmol),p-H2bdc(16.6 mg,0.1 mmol)and L(37.2 mg,0.1 mmol)was dissolved in 5 mL of DMF/H2O(4∶1,V/V).The final mixture was placed in a Parr Teflon-lined stainless steel vessel(15 mL)under autogenous pressure and heated at 95℃for three days.Colorless block crystals were collected. The yield of the reaction was ca.45%based on L ligand.Anal.Calcd.for C44H32CdN8O8S2(%):C,54.03; H,3.27;N,11.46.Found(%):C,54.09;H,3.20;N, 11.40.IR(KBr,cm-1):3 430(s),3 091(s),1 547(s), 1 478(s),1 416(m),1 300(s),1 256(s),1 195(m),1 128 (w),1 055(s),956(m),902(w),831(s),735(m),722(m), 658(m),545(w),505(w).
1.3Single crystal X-ray crystallography
Two block single crystals with dimensions of 0.24 mm×0.20 mm×0.18 mm for 1 and 0.18 mm×0.16 mm×0.12 mm for 2 were mounted on glass fibers for measurement,respectively.The data collections were carriedoutonaBrukerSmartApexⅡCCD diffraction(Mo Kα,λ=0.071 03 nm).The diffraction data were integrated using SAINT program[12],which was also used for the intensity correction for theLorentzandpolarizationeffects.Semi-empirical absorption corrections were applied using SADABS program[13].The structures were solved by direct methods,and all of the non-hydrogen atoms were refined anisotropically on F2by the full-matrix leastsquares technique using SHELXL-97 crystallographic software package[14].The hydrogen atoms except thoseof water molecules were generated geometrically and refined isotropically using the riding model.The details of the crystal parameters,data collection,and refinements for the compounds are listed in Table 1, and selected bond lengths and angles are summarized in Table 2.
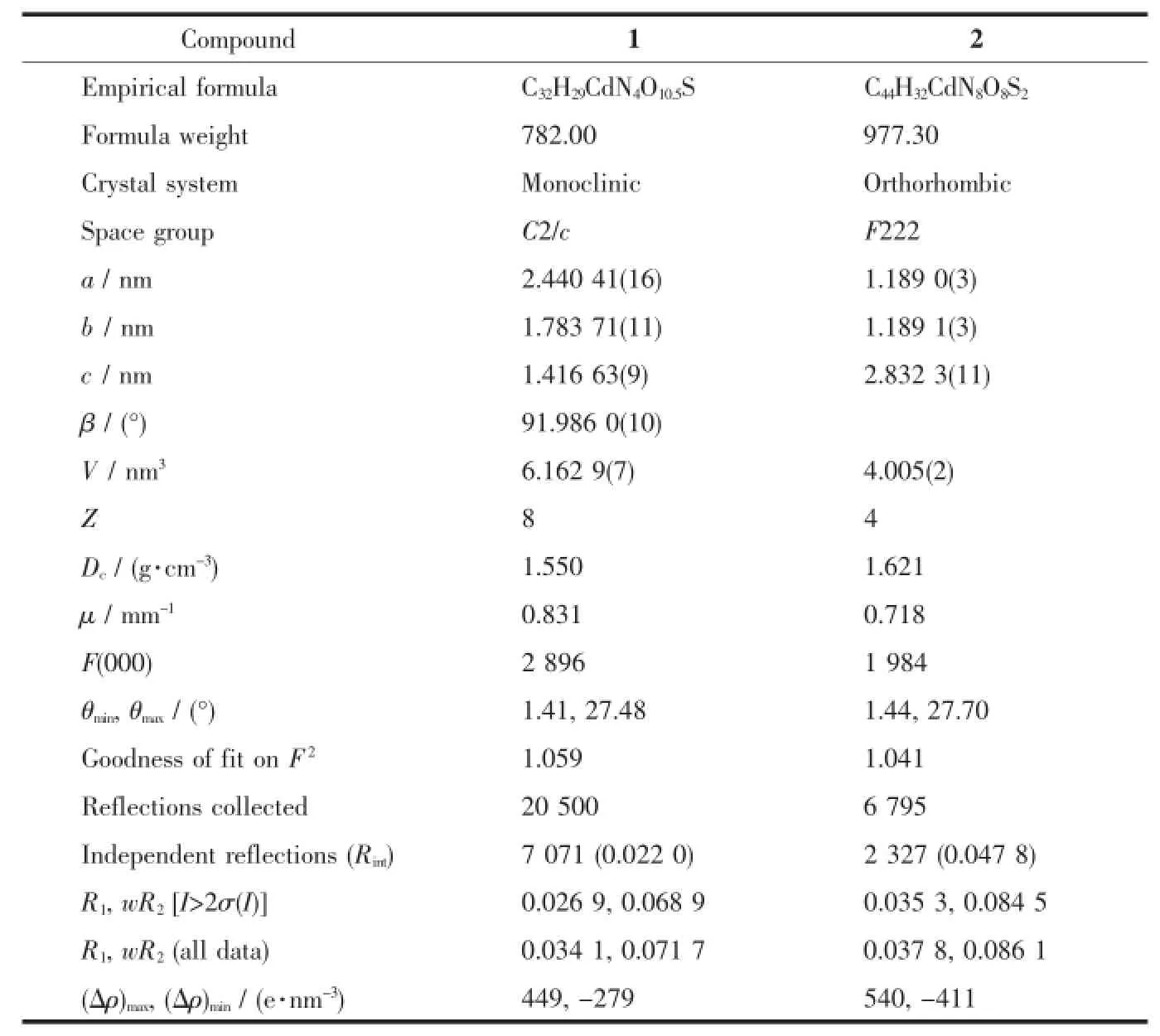
Table 1Crystallographic data for compounds 1 and 2
CCDC:1451100,1;1451101,2.
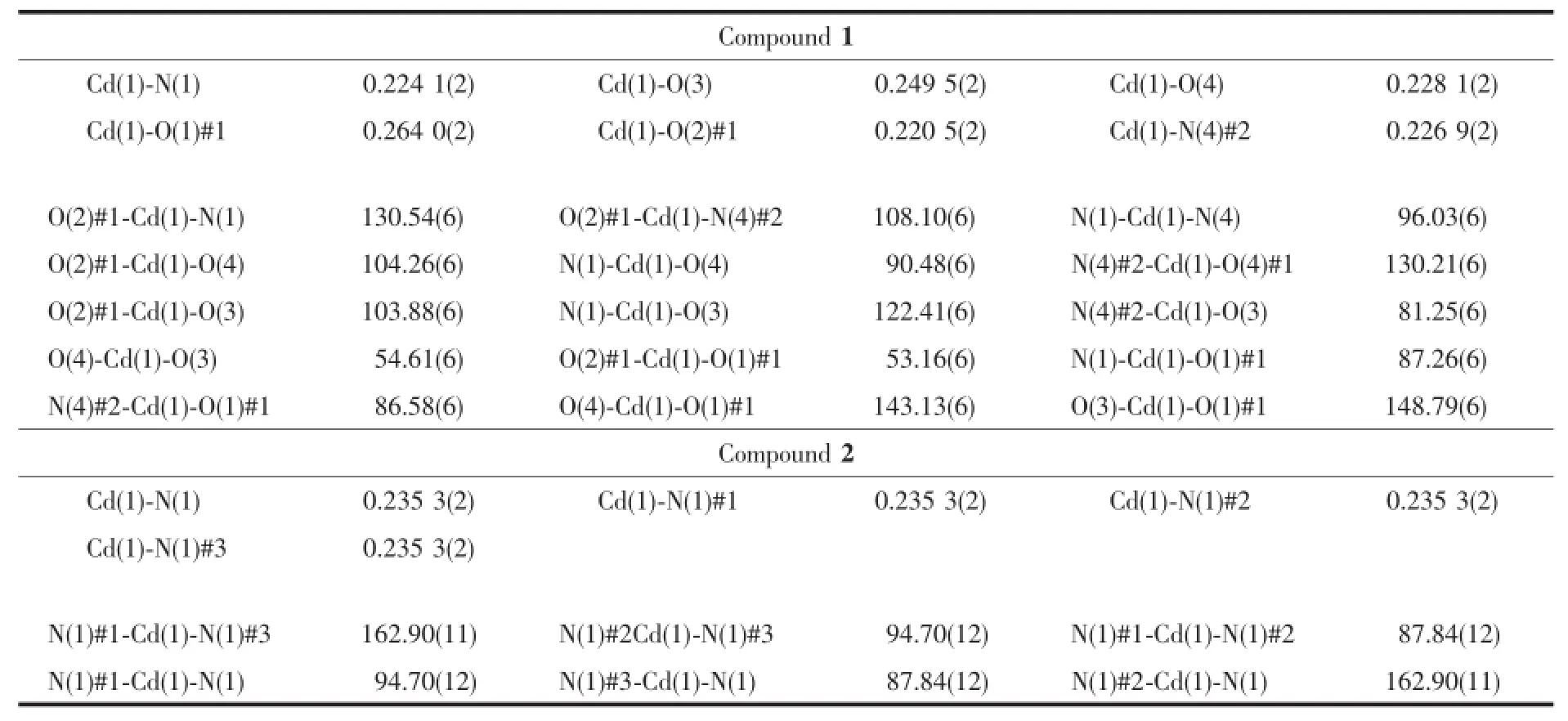
Table 2Selected bond distances(nm)and bond angles(°)of compounds 1 and 2
2 Results and discussion
2.1Structures description
2.1.1Crystal structure analysis of{[Cd(L)(oba)]·3.5H2O}n(1)
Single-crystal X-ray structural analysis reveals that 1 crystallizes in the monoclinic crystal system of C2/c space group.As shown in Fig.1,the asymmetric unit contains one Cd(Ⅱ)cation,one L ligand,one deprotonated 4,4′-oxydibenzoic acid and three point five water molecules,which was removed by the SQUEEZE routine in PLATON[15].Each Cd(Ⅱ)atom is surrounded by four Oatomsfromtwodifferent carboxylate groups oftwooba2-ligandsandtwo nitrogen atoms from two different L ligands.The Cd-O/N bond distances fall in the normal range found in other Cd compounds[16-17].The oba2-anions link Cd(Ⅱ)cations to form one infinite 1D linear chain with an Cd…Cd distance of 1.511 4 nm and Cd…Cd…Cd angle of 180.00°.Similarly,the neighboring Cd(Ⅱ)cations are linked by L ligands via Cd-N bonds, affordinganot-so-commonmeso-helicalmotif,a special type of helical chain where right-and lefthanded helices are formed in equal amounts within a single helical chain(Fig.2).Finally,these two kinds of 1D chains are cross-linked by sharing the Cd(Ⅱ)cations into a three-dimensional network(Fig.3).Cd(Ⅱ)can be regarded as a network node,and each node connects to four adjacent ones.Thus the 3D coordination polymer lattice for 1 can be characterized as a 4-connected cds network with the point symbol of {65·8}.The potential voids are large enough to be filled via mutual interpenetration of an independent equivalent framework,generating a 2-fold interpenetrating 3D architecture(Fig.4).
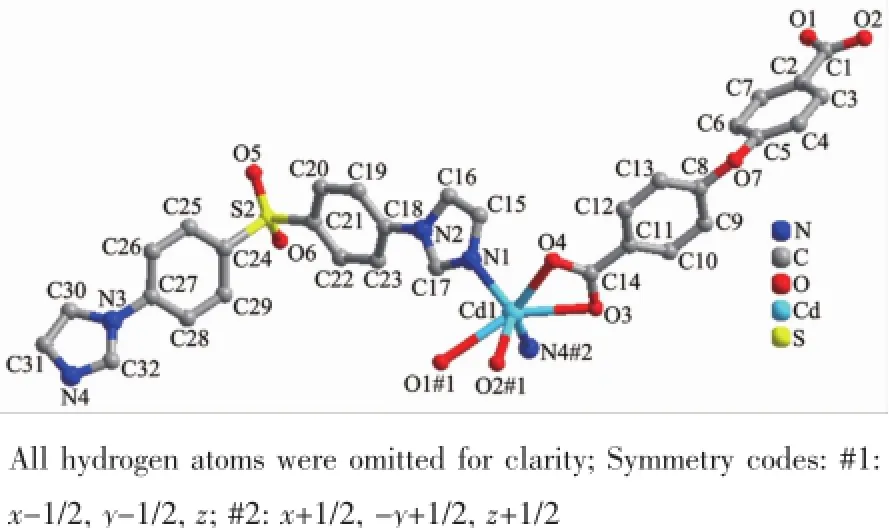
Fig.1Coordination environment of the Cd(Ⅱ)cation in 1

Fig.2View of the infinite meso-helical chain formed by L ligands linked through Cd ions
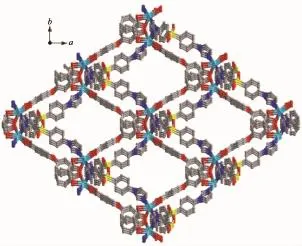
Fig.3Schematic view of the 3D network of 1 along the c axis
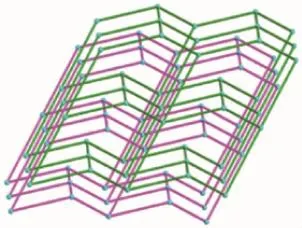
Fig.4Two-fold interpenetrating 3D cds-type topology of 1
2.1.2Crystal structure analysis of{[Cd(L)2](p-bdc)}n(2)
Single-crystal X-ray structural analysis reveals that 2 crystallizes in orthorhombic crystal system of the F222 space group.The asymmetric unit contains one Cd(Ⅱ)cation,two L ligands,one singly deprotonated p-bdc2-anion in free form.The coordination environment around the Cd(Ⅱ)cation is exhibited in Fig.5. Each Cd(Ⅱ)is coordinated by four N donors from four L ligands with a slightly distorted square-planar geometry,which have rarely reported for Cd(Ⅱ)coordination polymers.The Cd-N distances are 0.235 3(3) nm,and the N-Cd-N angles range from 87.84(14)°to162.90(13)°.These are similar to values found in other Cd(Ⅱ)compounds.As shown in Fig.6,each Cd(Ⅱ)atoms are linked by L ligands to produce a 2D layer. Topological analysis reveals that each Cd(Ⅱ)center can be regarded as the four-connected nodes and L ligands as likers.Thus,the 2D layer can be regarded as a 4-connected sql network with the point symbol of {44·62}(Fig.7).
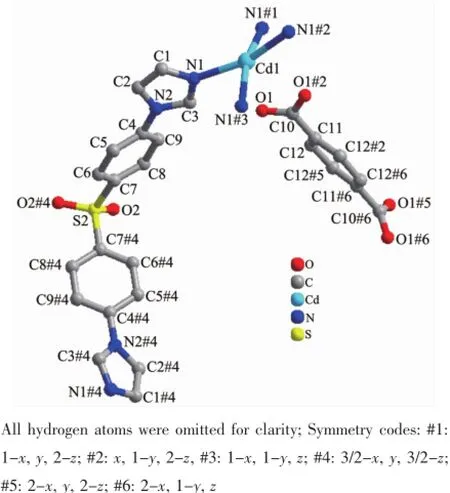
Fig.5Coordination environment of the Cd(Ⅱ)cation in 2
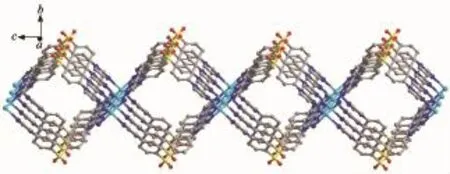
Fig.62D network of 2 along the a axis
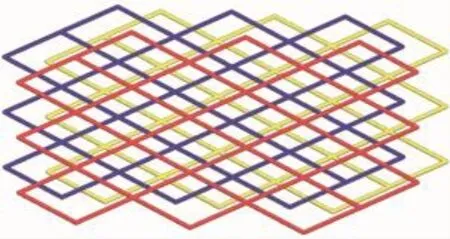
Fig.7Schematic view of sql topology of 2
2.2Thermal stability and powder X-ray diffraction(PXRD)
PowderX-raydiffractionanalysis(PXRD) experiments were carried out for 1 and 2 at room temperature to characterize their purity.As shown in Fig.8,the measured peak positions closely match the simulated peak positions,indicative of pure products.
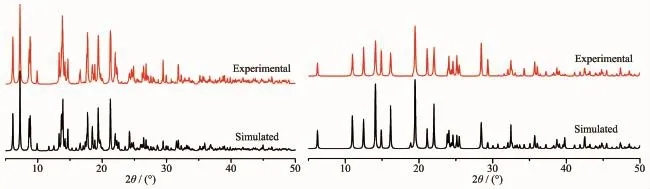
Fig.8PXRD patterns of compounds 1(left)and 2(right)
To examine the thermal stabilities of compounds 1 and 2,their thermal decomposition behavior was investigatedfrom20to750℃undernitrogen atmosphere(Fig.9).The TGA curve of compound 1 indicate that there is a weight loss of approximate 8.24%between 130 and 160℃,corresponding to the loss of release of lattice water molecule(Calcd. 8.06%),and the framework collapses at 430℃.The TGA study of compound 2 indicates no obvious weight loss from 20 to 240℃,suggesting that the frameworks are thermally stable.
2.3Photoluminescence properties
Thesolid-stateluminescentpropertiesof compounds 1 and 2 have been investigated at room temperature under the same conditions.The emission spectra of the compounds are depicted in Fig.10.It can be seen that the emission bands of compounds were observed at 416 nm(λex=342 nm)for 1 and 432 nm(λex=350 nm)for 2,respectively.The enhancement in the fluorescence intensity is realized compared withthe free L,H2oba and p-H2bdc ligands,which have almost no fluorescence properties.The enhancement of luminescence in d10compounds may be attributed to the ligation of the metal center to the ligands.The coordination effect increases the rigidity of the ligands and thus reduces the loss of energy through a radiationless pathway[18].
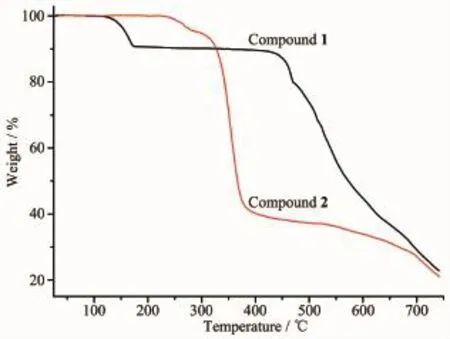
Fig.9TGA curves of compounds 1 and 2
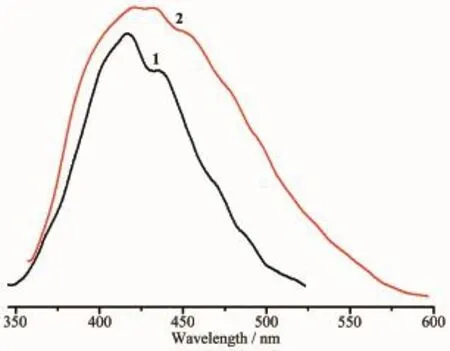
Fig.10Solid-state photoluminescent spectra for 1 and 2
3 Conclusions
In summary,we have successfully synthesized twonewcoordinationpolymers,whichwere constructed from a simple“V-shaped”ligand in the presenceofco-ligandsandCdionsunder hydrothermal conditions.The ancillary ligands play a significant role in the structure of the final products. Additionally,compounds1 and 2 display strong fluorescence and better thermal stability.
References:
[1]Lin Z J,Huang Y B,Liu T F,et al.Inorg.Chem.,2013,52: 3127-3132
[2]Li P,He Y,Guang J,et al.J.Am.Chem.Soc.,2014,136: 547-549
[3]Zhu Y,Wang Y M,Zhao S Y,et al.Inorg.Chem.,2014,53: 7692-73699
[4]Wang L H,Zeng Y,Shen A G,et al.Chem.Commun.,2015, 51:2052-2055
[5]He C B,Lu K D,Liu D M,et al.J.Am.Chem.Soc.,2014, 136:5181-5184
[6]Zhang C L,Qin L,Shi Z Q,et al.Dalton Trans.,2015,44: 4238-4245
[7]Qin L,Zheng M X,Guo Z J,et al.Chem.Commun.,2015, 51:2447-2449
[8]Zheng M X,Gao X J,Zhang C L,et al.Dalton Trans.,2015, 44:4751-4758
[9]Wang T,Zhang C L,Ju Z M,et al.Dalton Trans.,2015,44: 6926-6935
[10]Li X J,Yu Z J,Guan T N,et al.Cryst.Growth Des.,2015, 15:278-290
[11]Hu J S,Shang Y J,Yao X Q,et al.Cryst.Growth Des., 2010,10:4135-4142
[12]SMART and SAINT,Siemens Analytical X-ray Instrument Inc.,Madison,WI,1996.
[13]Sheldrick G M.SADABS,Program for Empirical Absorption Correction of Area Detector Data,University of G?ttingen, Germany,1996.
[14]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,Universirty of G?ttingen,Germany,1997.
[15]Spek A L.Acta Crystallogr.A,1990,A46:194-201
[16]Yi F Y,Li J P,Wu D,et al.Chem.Eur.J.,2015,1:1-9
[17]Wu Y L,Yang G P,Zhou X,et al.Dalton Trans.,2015,44: 10385-10391
[18]Wang Z J,Qin L,Zhang X,et al.Cryst.Growth Des.,2015, 15:1303-1310
Syntheses,Crystal Structures and Fluorescence Properties of Two Compounds Constructed by Aromatic Carboxylates and 4,4′-Bis(imidazol-l-yl)-phenyl Sulphone
XU Han1,2
(1School of Chemistry and Chemical Engineering,Huangshan University,Huangshan,Anhui 245041,China)
(2State Key Laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,
Collaborative Innovation Center of Advanced Microstructures,Nanjing University,Nanjing 210023,China)
Two coordination polymers,namely{[Cd(L)(oba)]·3.5H2O}n(1)and{[Cd(L)2](p-bdc)}n(2)have been obtained by the reaction of cadmium nitrate,4,4′-bis(imidazol-l-yl)-phenyl sulphone(L)with two aromatic carboxylic acids,4,4′-oxydibenzoic acid(H2oba),terephthalic acid(p-H2bdc).They were characterized by IR, elemental analysis and X-ray diffraction.Compound 1 exhibits 4-connected 2-fold interpenetrating 3D networks with the point symbol of{65·8}.Compound 2 can be characterized as a 4-connected sql tetragonal planar network with the point symbol of{44·62}.The luminescent properties of two compounds have also been investigated. CCDC:1451100,1;1451101,2.
Cd(Ⅱ)coordination polymer;imidazole ligand;crystal structure;photoluminescence
O614.24+2
A
1001-4861(2016)08-1481-06
10.11862/CJIC.2016.194
2016-05-03。收修改稿日期:2016-07-15。
安徽高校省級自然科學研究基金(No.KJHS2016B10)資助項目。
E-mail:xuhannju@163.com

