兩個基于β-二酮席夫堿配體構(gòu)筑的銀配合物的合成、晶體結(jié)構(gòu)和熒光性質(zhì)
張奇龍 徐紅 馮廣衛(wèi) 黃亞勵(貴州醫(yī)科大學化學教研室,貴陽550004)
兩個基于β-二酮席夫堿配體構(gòu)筑的銀配合物的合成、晶體結(jié)構(gòu)和熒光性質(zhì)
張奇龍*徐紅馮廣衛(wèi)黃亞勵
(貴州醫(yī)科大學化學教研室,貴陽550004)
在常溫的條件下,分別將2個雙β-二酮席夫堿配體與銀鹽進行配位反應得到2個銀配合物,{[Ag(L1)](SbF6)}n(1)和[Ag2(L2)2] (BF4)2(2)(L1=1,3-bis(4-methylamino-pentan-2-one)phenyl,L2=1,3-bis(3-methylamino-1-phenyl-butan-1-one)phenyl),并通過元素分析、紅外光譜、粉末X射線衍射分析、單晶X射線衍射等對其結(jié)構(gòu)進行了表征。結(jié)構(gòu)分析表明,在配合物1中,銀離子與配體L1中的2個碳原子和2個氧原子配位,形成一維鏈狀結(jié)構(gòu),而配合物2中銀離子與L2配體中的2個氧原子和2個碳原子配位,最終得到雙核二聚體結(jié)構(gòu)。化合物1和2都通過陰離子與結(jié)構(gòu)單元之間的C-H…F作用,最終形成三維超分子結(jié)構(gòu)。此外,我們還研究了化合物1、2以及配體的熒光性質(zhì)。
銀配合物;晶體結(jié)構(gòu);熒光性質(zhì);氫鍵作用
0 Introduction
As an outstanding representative of inorganicorganic hybrid materials,the coordination complexes have drawn much attention in last two decades for their diverse structures as well as tunable applications[1-5].The acetylacetone Schiff bases,which holding semi-enclosed structure,generally contain carbonyl groups and secondary nitramine groups together,can act as the hydrogen bonds donors and acceptors in theassembly of coordination complexes[6-8].Owing to their strong coordination ability and multiple coordination modes,the acetylacetone Schiff bases have been widelyusedintheassemblyofcoordination complexes,which show potential applications in the field of gas storage and separation,heterogeneous catalysis,magnetism,luminescence,and so on[9-10].
Inspired by the above-mentioned points,we were interested in the syntheses and properties of bis(βdiketone)Schiff bases coordination complexes and reported two novel silver coordination complexes, {[Ag(L1)](SbF6)}n(1)and[Ag2(L2)2](BF4)2(2)(L1=1,3-bis(4-methylamino-pentan-2-one)phenyl,L2=1,3-bis(3-methylamino-1-phenyl-butan-1-one)phenyl).In complex 1,the center Ag(Ⅱ)ions connected with two C atoms and two O atoms to form a one-dimensional chain, while the complex 2 is a binuclear silver complex. Moreover,the luminescence investigation shows that complexes 1 and 2 emitted green and blue fluorescence, respectively.
1 Experimental
1.1Materials and methods
Allchemicalswerecommerciallyobtained without further purification.IR spectra were measured on a Nicolet 740 FTIR Spectrometer at the range of 400~4 000 cm-1.Elemental analyses were carried out on a CE instruments EA 1110 elemental analyzer. Fluorescence spectra were performed on a Hitachi F-4500 fluorescence spectrophotometer at room temperature.Powder X-ray diffraction(PXRD)analyses were performed on an X-ray diffractometer(D/max 2500 PC, Rigaku)with Cu Kα radiation(λ=0.154 06 nm).
1.2Synthesis
1.2.1Synthesis of{[Ag(L1)](SbF6)}n(1)
AgSbF6(34.4 mg,0.1 mmol)in ethanol(20 mL) was added dropwise with stirring to L1(30.4 mg,0.1 mmol)in ethanol(20 mL)and the mixture was stirred at room temperature for several days.Slow evaporation of this solution yielded colorless block crystals that proved suitable for X-ray analysis.The precipitate that formed was collected by filtration,and dried at room temperature to give 1 in 48%yield based on Ag. Anal.Calcd.for C18H24AgF6N2O2Sb(%):C,33.57;H, 3.76;N,4.35.Found(%):C,33.31;H,3.58;N,4.41. IR(KBr pellet,cm-1):3 438(s),3 149(m),1 617(vs), 1 519(s),1 401(s),1 303(m),1 169(w),1 074(s),808 (m),592(m),472(m).
1.2.2Synthesis of[Ag2(L2)2](BF4)2(2)
The synthesis of 2 is similar to 1 using AgBF4(19.4 mg,0.1 mmol)and L2(42.8 mg,0.1 mmol)instead of AgSbF6and L1.Colorless block.Yield:54%yield based on Ag.Anal.Calcd.for C56H56Ag2B2F8N4O4(%): C,54.31;H,4.56;N,4.52.Found(%):C,54.37;H, 4.61;N,4.56.IR(KBr pellet,cm-1):3 440(s),3 129 (m),1 587(vs),1 544(m),1 436(m),1 393(s),1 319 (m),1 223(w),1 061(vs),1 008(m),857(w),782(m), 707(m),569(w),523(w).
1.3X-ray crystallography
The single-crystal X-ray diffraction was performed on a Bruker Smart ApexⅡCCD diffractometer. Intensities of reflections were measured using graphite -monochromatized Mo Kα radiation(λ=0.071 073 nm) at 296(2)K with the data collection θ ranging from 1.67°to 26°for 1,and at 293(2)K with the data collection θ ranging from 1.73°to 25.01°for 2, respectively.The structurewassolvedbydirect methods using the SHELXS program of the SHELXTL package and refined with SHELXL[11].Anisotropic thermal factors were assigned to all the non-hydrogen atoms.H atoms attached to C were placed geometrically and allowed to ride during subsequent refinement with an isotropic displacement parameter fixed at 1.2 times Ueqof the parent atoms.H atoms bonded to O or N atoms were first located in difference Fourier maps and then placed in the calculated sites and included in the refinement.Crystallographic data parameters for structural analyses are summarized in Table 1.Selected bond lengths and angles for complex 1 and 2 are listed in Table 2 and Table 3,respectively.
CCDC:1469231,1;1469232,2.
2 Results and discussion
2.1Crystal structure of{[Ag(L1)](SbF6)}n(1)
Complex 1 crystallizes in the triclinic space group P1,and its asymmetric unit contains onecrystallographically independent Ag(Ⅱ)ion,one L1ligand,and one SbF6-anion.As shown in Fig.1,the Ag(Ⅱ)ion is four-coordinated by two oxygen atoms (O1iiand O2i),and two carbon atoms(C10 and C15) from three different L1ligands.The central Ag(Ⅱ)ion located in a distorted AgC2O2tetrahedral coordination geometry,with the τ4=0.70(τ4=[360°-(α+β)]/141°,in which α and β are the two largest bond angles in the four-coordinate complex)[12].Besides,the Ag-C bond lengths are 0.234 0(9)and 0.236 7(1)nm,while the Ag-Odistancesare 0.237 1(4)and 0.274 4(9)nm (Table 2).Except the weak Ag1-O2iiinteractions,other bond lengths are in the normal ranges of Ag-O bonds in silver complexes[13].In the complex 1,two acetylacetone units of the L1ligand bridge three Ag(Ⅱ)centres through Oacetylacetoneand Cacetylacetone,extending in the direction of a axis,leaving a one-dimensional polymeric chain finally,with the nearest Ag…Ag distances being 0.527 7(6)and 0.565 3(2)nm(Fig.2). The one-dimensional polymeric chains interacted with the SbF6-anions through C-H…F hydrogen bonds (Table 4),finally gave a three-dimensional supramolecular network(Fig.3).
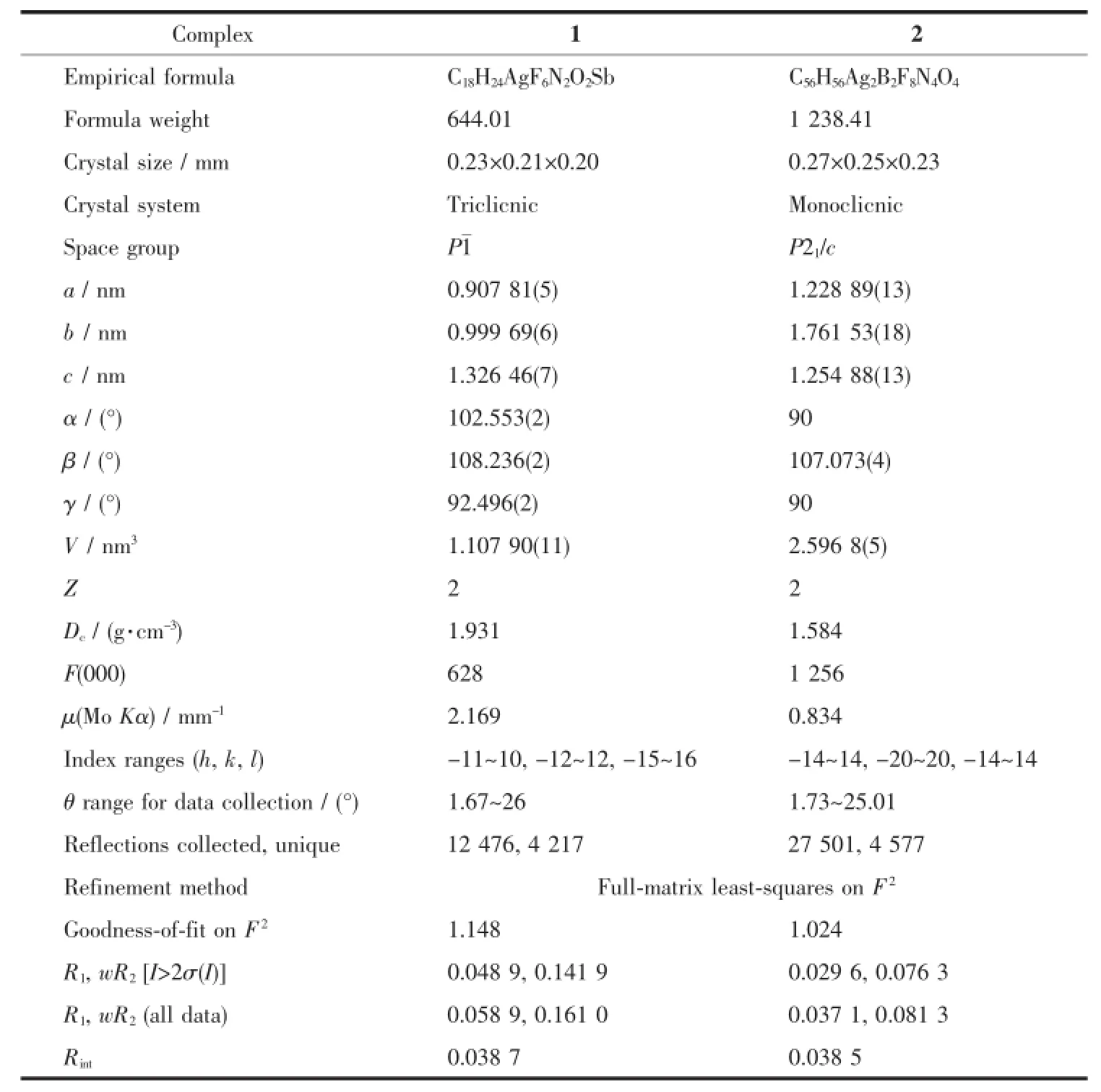
Table 1Crystal structure parameters of complexes 1 and 2
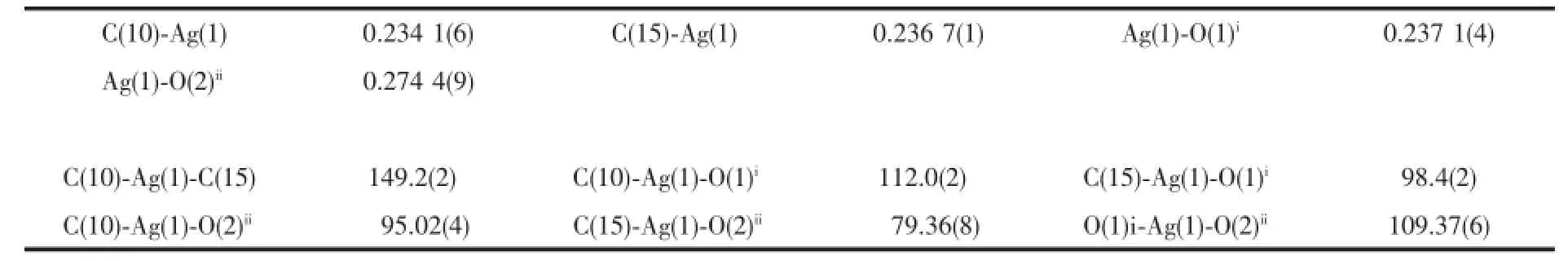
Table 2Selected bond lengths(nm)and bond angles(°)for 1
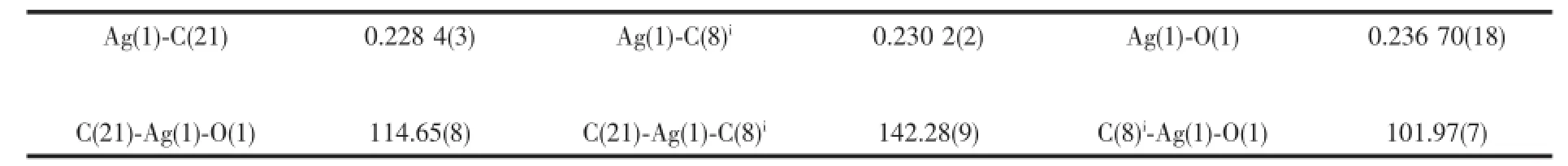
Table 3Selected bond lengths(nm)and bond angles(°)for 2
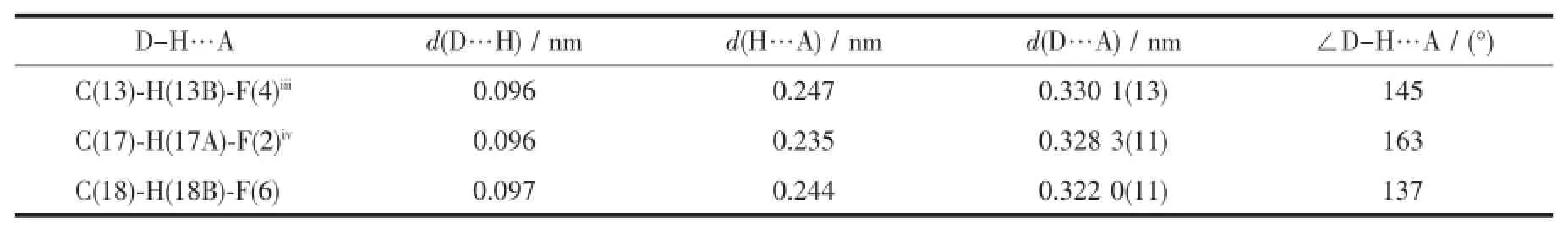
Table 4Hydrogen bond geometry of complex 1
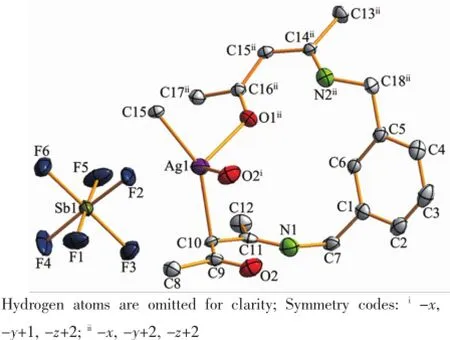
Fig.1Asymmetric unit of complex 1 with 30% probability level
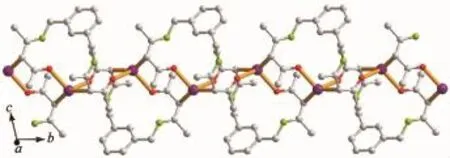
Fig.2One-dimensional polymeric chain structure of complex 1
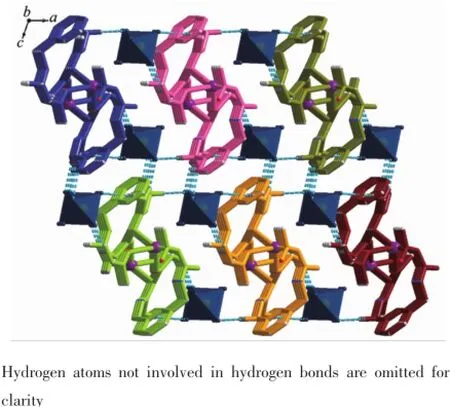
Fig.3Three-dimensional supramolecular framework of complex 1
2.2Crystal structure of[Ag2(L2)2](BF4)2(2)
In complex 2,the steric effects of phenyl group in L2ligand make it impossible to form a similar 1D polymeric chain structure like 1.Complex 2 crystallizes in the monoclinic space group P21/c.There are one crystallographically independent silver ion,one L2ligand,and one BF4-anion in the asymmetric unit of 2 (Fig.4).The central Ag(Ⅱ)ion is three-coordinated by one oxygen atom(O1),and two carbon atoms(C21 and C8i)from two different L2ligands.And the Ag-C bond lengths are 0.228 3(7)and 0.230 1(6)nm,the Ag-O distance is 0.236 7(0)nm,respectively(Table 3).The units interacted with the adjancent ones and the BF4-anions through the C-H…F andC-H…O hydrogen bond interactions(Table 5),and finally give a stable three-dimensional supramolecular architecture (Fig.5).
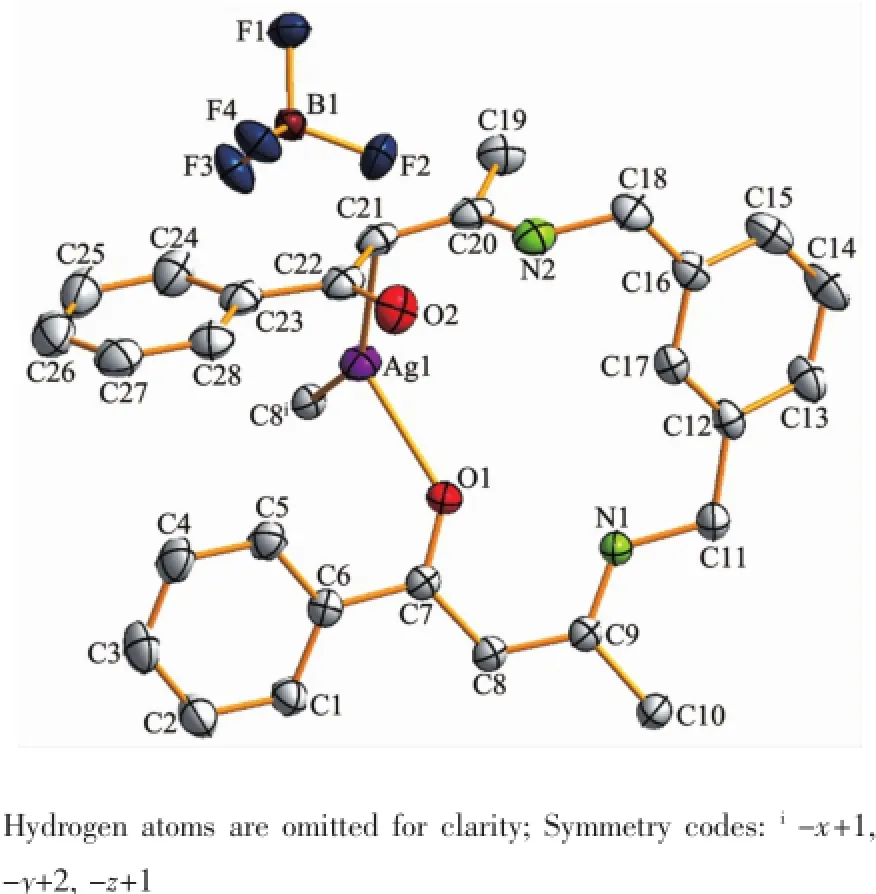
Fig.4Molecular structure of complex 2 with 30% probability level
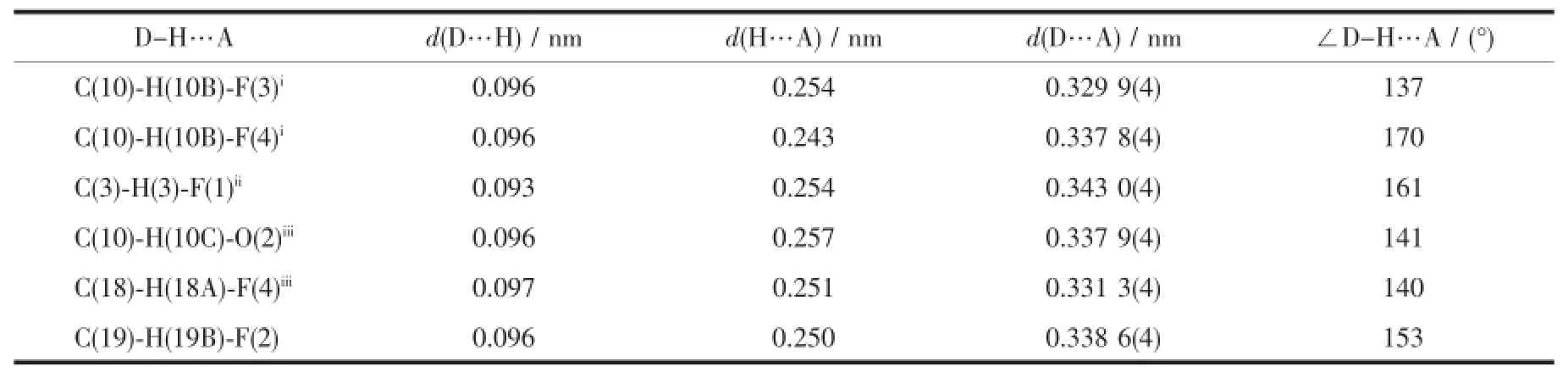
Table 5Hydrogen-bond geometry of complex 2
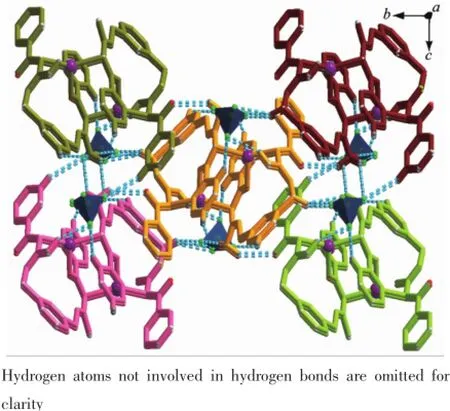
Fig.5Three-dimensional supramolecular framework ofcomplex 2
2.3Powder X-ray diffraction
In order to check the phase purity of the complexes,the PXRD patterns of title complexes were determined at room temperature.As shown in Fig.6, the peak positions of the simulated and experimental PXRD patterns are in agreement with each other, demonstrating the good phase purity of the complexes. The dissimilarities in intensity may be due to the preferredorientationofthecrystallinepowder samples.
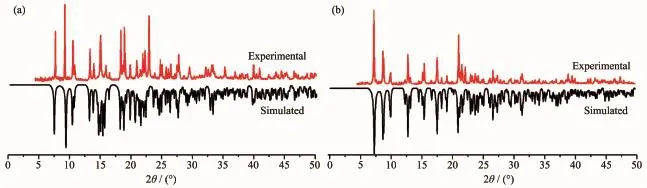
Fig.6PXRD patterns of 1(a)and 2(b)
2.4Photoluminescent properties
Many coordination complexes have been extensively studied due to their potential applications as luminescent materials[14-16].The solid-state luminescent properties of the complexes and the ligands were investigated at room temperature.The photoluminescence spectra of two organic ligands and complex 1 and 2 are shown in Fig.7.The L1and L2display photoluminescence with max emission peak at 518 nm (λex=488 nm),and 483 nm(λex=398 nm),respectively. It can be presumed that the peak originate from the π*→n or π*→π transitions[17-18].It can be observed that intense emissions occur at 514 nm(λex=486 nm) for 1,and 481 nm(λex=396 nm)for 2,respectively. The resemblance of the emissions between the organic ligandsandtheirbasedcoordinationcomplexes indicates that the emissions of the two complexes are probably attributed to the π→π*transitions[19].
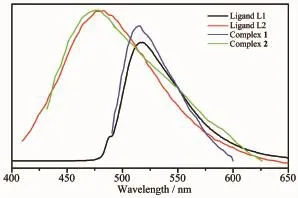
Fig.7Solid-state emission spectra of 1 and 2,and two free ligands at room temperature
3 Conclusions
In summary,two bis(β-diketone)Schiff bases silver coordinationcomplexes,{[Ag(L1)](SbF6)}n(1),and [Ag2(L2)2](BF4)2(2)have been reported.In complex 1, the center Ag(Ⅱ)ions connected with two C atoms and two O atoms to form a one-dimensional chain,while the complex 2 is a binuclear silver complex.Expanded by the weak C-H…F interactions between the units and anions and C-H…O hydrogen bonds between the adjacent units,two complexes formed three-dimensional supramolecularframeworkfinally.Moreover,the luminescence investigation shows that complexes 1 and 2 emitted green and blue fluorescence,respectively.
References:
[1]Zhang J P,Zhang Y B,Lin J B,et al.Chem.Rev.,2012,112: 1001-1033
[2]Chen X Y,Huang R B,Zheng L S,et al.Inorg.Chem., 2014,53:5246-5252
[3]Xue M,Lü Y C,Sun Q Q,et al.Cryst.Growth Des.,2015,15 (11):5360-5367
[4]Lin G Q,Ding H M,Yuan D Q,et al.J.Am.Chem.Soc., 2016,138(10):3302-3305
[5]Jiang J C,Zhao Y B,Yaghi O M.J.Am.Chem.Soc.,2016, 138(10):3255-3265
[6]Cherutoi J K,Sandifer J D,Pokharel U R,et al.Inorg.Chem., 2015,54(16):7791-7802
[7]ZHANG Qi-Long(張奇龍),WANG Huan-Yu(王煥宇),JIANG Feng(江峰),et al.Chinese J.Inorg.Chem.(無機化學學報), 2016,32:464-468
[8]Zhang Q L,Hu P,Zhao Y,et al.J.Solid State Chem.,2014, 210:178-187
[9]Zhang Q,Ma J P,Wang P,et al.Cryst.Growth Des.,2008,8 (7):2581-2587
[10]Pariya C,Fronczek F R,Maverick A W.Inorg.Chem.,2011, 50(7):2748-2753
[11]Sheldrick G M.SHELXS-97,Program for Crystal Structure Refinement,University of G?ttingen,G?ttingen,Germany, 1997.
[12]Fan L M,Fan W L,Li B,et al.CrystEngComm,2015,17: 9413-9422
[13]ZHANG Qi-Long(張奇龍),FENG Guang-Wei(馮廣衛(wèi)),HU Peng(胡鵬).Chinese J.Inorg.Chem.(無機化學學報),2014, 30:2433-2439
[14]Chen D M,Ma X Z,Shi W,et al.Cryst.Growth Des.,2015, 15(8):3999-4004
[15]ZHANG Qi-Long(張奇龍).Chinese J.Inorg.Chem.(無機化學學報),2015,31:2213-2220
[16]Wang W,Yang J,Wang R M,et al.Cryst.Growth Des., 2015,15(6):2589-2592
[17]LI Guo-Feng(李國峰),LI Xiu-Mei(李秀梅),JI Jian-Ye(紀建業(yè)),et al.Chinese J.Inorg.Chem.(無機化學學報),2014, 30:1947-1953
[18]Fan L M,Zhang X T,Li D,et al.CrystEngComm,2013,15: 349-355
[19]Zhang X T,Fan L M,Fan W L,et al.CrystEngComm,2015, 17:6681-6692
Syntheses,Structures and Luminescent Properties of Two Silver Coordination Complexes Based on Bis(β-diketone)Schiff Bases
ZHANG Qi-Long*XU HongFENG Guang-WeiHUANG Ya-Li
(Department of Chemistry,Guizhou Medical University,Guiyang 550004,China)
Two silver complexes,namely{[Ag(L1)](SbF6)}n(1)and[Ag2(L2)2](BF4)2(2),have been obtained by the reaction of silver salts with two different bis(β-diketone)Schiff bases(L1=1,3-bis(4-methylamino-pentan-2-one) phenyl,and L2=1,3-bis(3-methylamino-1-phenyl-butan-1-one)phenyl)in the ethanol solution.In complex 1,the center Ag(Ⅱ)ions connected with two C atoms and two O atoms to form a one-dimensional chain,while the complex 2 is a binuclear silver complex.Expanded by the weak C-H…F interactions between the units and anions and C-H…O hydrogen bonds between the adjacent units,three-dimensional supramolecular structures of two complexes formed finally.Moreover,the luminescent properties of two silver complexes and their based organic ligands have been investigated.CCDC:1469231,1;1469232,2.
silver complex;crystal structure;fluorescent property;hydrogen bonds
O614.122
A
1001-4861(2016)08-1421-06
10.11862/CJIC.2016.151
2016-03-18。收修改稿日期:2016-05-03。
貴州省科技計劃課題(黔科合LH字[2014]7090)、貴州省教育廳自然科學研究項目(黔教合KY字[2015]415號)和貴陽市科技局國家自然科學基金培育項目(No.GY2015-41)資助。
*通信聯(lián)系人。E-mail:gzuqlzhang@126.com
- 無機化學學報的其它文章
- 4,4′-聯(lián)吡啶橋聯(lián)的三個銅有機膦酸配合物的合成、結(jié)構(gòu)及磁性
- 由芳香羧酸和4,4′-二(1-咪唑基)苯砜為配體構(gòu)筑的兩個配合物的合成、晶體結(jié)構(gòu)及熒光性質(zhì)
- 三個包含酰胺型配體銅/鋅配合物的合成、結(jié)構(gòu)及熒光性質(zhì)
- 由4-甲基-1,2,3-噻二唑-5-甲酸構(gòu)筑的銅配合物的合成、晶體結(jié)構(gòu)及與DNA作用
- 以5-甲基-3-吡唑甲酸為配體的鈷(Ⅱ)、鎳(Ⅱ)配合物的合成、晶體結(jié)構(gòu)和性質(zhì)
- 特戊酸和含氮配體構(gòu)筑的Ln(Ⅱ)配合物的結(jié)構(gòu)、熱穩(wěn)定性和熒光性質(zhì)

