哮喘新生兒臍帶血炎性因子水平變化及其臨床意義
馬 超,任少敏
?
·診治分析·
哮喘新生兒臍帶血炎性因子水平變化及其臨床意義
馬 超,任少敏
目的分析哮喘新生兒臍帶血炎性因子水平變化及其臨床意義。方法選取2012—2015年內蒙古醫科大學附屬醫院收治的哮喘孕婦60例作為研究組,另選取同期內蒙古醫科大學附屬醫院收治的健康孕婦60例作為對照組。比較兩組新生兒臍帶血炎性因子〔白介素18、白介素5、白介素10、白介素1、白介素6、腫瘤壞死因子α(TNF-α)〕水平、新生兒哮喘發生情況及研究組哮喘新生兒與無哮喘新生兒臍帶血炎性因子水平。結果研究組新生兒臍帶血白介素18、白介素5、白介素1、白介素6、TNF-α水平高于對照組,臍帶血白介素10水平低于對照組(P<0.05)。研究組新生兒哮喘發生率高于對照組(P<0.05)。研究組哮喘新生兒臍帶血白介素18、白介素5、白介素1、白介素6、TNF-α水平高于無哮喘新生兒,臍帶血白介素10水平低于無哮喘新生兒(P<0.05)。結論哮喘新生兒臍帶血炎性因子水平較高,早期檢測新生兒臍帶血炎性因子水平并采取有針對性的干預措施有助于減少新生兒哮喘的發生。
哮喘;嬰兒,新生;胎血;炎性因子
馬超,任少敏.哮喘新生兒臍帶血炎性因子水平變化及其臨床意義[J].實用心腦肺血管病雜志,2016,24(9):81-83.[www.syxnf.net]
MA C,REN S M.Change and clinical significance of umbilical cord blood inflammatory cytokines levels in neonates with asthma[J].Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease,2016,24(9):81-83.
世界范圍內,新生兒哮喘的發病率均較高[1],其受環境和遺傳因素影響[2]。環境因素主要包括病毒、細菌感染,而遺傳因素在新生兒哮喘中的作用機制尚不明確。MENDOLA等[2]研究表明,新生兒哮喘與產婦哮喘關系密切。新生兒父母一方有哮喘病史,則新生兒哮喘發病率是正常新生兒的3倍;若父母雙方均有哮喘病史,則新生兒哮喘的發病率是正常新生兒的10倍。新生兒哮喘是成人哮喘的主要危險因素[3]。哮喘嚴重影響患者的生活質量,增加了社會的經濟負擔[4-5]。有研究表明,哮喘患者體內炎性因子(白介素1、白介素6等)水平異常[6-9]。臍帶是胎兒獲取營養的主要途徑,故推測異常水平的炎性因子可通過臍帶血而作用于胎兒,導致新生兒哮喘,但目前相關研究較少。本研究旨在分析哮喘新生兒臍帶血炎性因子水平變化及其臨床意義,現報道如下。
1 資料與方法
1.1納入與排除標準
1.1.1研究組納入標準:(1)足月分娩;(2)有哮喘;(3)自愿參與本研究;(4)分娩時哮喘處于緩解期;(5)年齡20~35歲。排除標準:(1)妊娠期間使用糖皮質激素者;(2)有原發性臟器功能不全者;(3)有妊娠性高血壓疾病者;(4)有子癇者;(5)有繼發性心功能不全者;(6)有其他慢性炎性疾病者;(7)有糖尿病、高血壓或高脂血癥病史者;(8)隨訪期間失訪者。
1.1.2對照組納入標準:(1)足月分娩;(2)自愿參與本研究;(3)年齡20~35歲。排除隨訪期間失訪者。
1.2一般資料選取2012—2015年內蒙古醫科大學附屬醫院收治的哮喘孕婦60例作為研究組,另選取同期內蒙古醫科大學附屬醫院收治的健康孕婦60例作為對照組。研究組孕婦年齡21~35歲,平均年齡(29.4±6.4)歲;配偶有哮喘病史者5例;胎齡37~42周,平均胎齡(38.6±0.8)周;剖宮產14例,自然分娩46例。對照組孕婦年齡22~35歲,平均年齡(29.0±6.0);配偶有哮喘病史者4例;胎齡37~42周,平均胎齡(38.8±0.8)周;剖宮產12例,自然分娩48例。兩組孕婦年齡(t=0.274)、配偶哮喘病史者所占比例(χ2=0.000)、胎齡(t=0.158)、分娩方式(χ2=0.196)比較,差異無統計學意義(P>0.05),具有可比性。所有孕婦簽署知情同意書,本研究經醫院倫理委員會審核批準。
1.3哮喘的診斷標準(1)反復發作性喘息、呼吸困難、胸悶或咳嗽;(2)哮喘發作時雙肺可聞及散在或彌漫性以呼氣相為主的哮鳴音,呼氣相變長;(3)治療后可完全緩解或自行緩解;(4)排除其他疾病引起的喘息、胸悶或咳嗽;(5)上述癥狀不典型者具備以下1項即可診斷:①支氣管激發試驗或運動試驗陽性,②支氣管擴張試驗陽性,③最大呼氣流量(PEF)日內變異率或晝夜波動率≥20%。
1.4觀察指標比較兩組新生兒臍帶血炎性因子〔白介素18、白介素5、白介素10、白介素1、白介素6、腫瘤壞死因子α(TNF-α)〕水平、新生兒哮喘發生情況及研究組哮喘新生兒與無哮喘新生兒臍帶血炎性因子水平。
1.5檢測方法采集臍帶血5 ml,采用酶聯免疫吸附法(ELISA)檢測臍帶血白介素18、白介素5、白介素10、白介素1、白介素6、TNF-α水平,試劑盒購自中國武漢博士德生物工程有限公司。
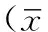
2 結果
2.1兩組新生兒臍帶血炎性因子水平比較研究組新生兒臍帶血白介素18、白介素5、白介素1、白介素6、TNF-α水平高于對照組,臍帶血白介素10水平低于對照組,差異有統計學意義(P<0.05、見表1)。
2.2兩組新生兒哮喘發生率比較研究組新生兒出現哮喘6例(10%);對照組新生兒無一例出現哮喘。研究組新生兒哮喘發生率高于對照組,差異有統計學意義(χ2=4.386,P=0.027)。
2.3研究組哮喘新生兒與無哮喘新生兒臍帶血炎性因子水平比較研究組哮喘新生兒臍帶血白介素18、白介素5、白介素1、白介素6、TNF-α水平高于無哮喘新生兒,臍帶血白介素10水平低于無哮喘新生兒,差異有統計學意義(P<0.05,見表2)。

Table 1Comparison of umbilical cord blood inflammatory cytokines levels of neonates between the two groups

組別例數白介素18白介素5白介素10白介素1白介素6TNF-α對照組60277.3±92.8106.7±13.529.7±6.676.0±18.285.9±19.781.0±19.8研究組60497.2±112.9151.4±24.519.5±5.5119.6±25.6112.4±27.2116.5±30.6t值11.65612.3949.20210.7576.1267.551P值0.0000.0000.0000.0000.0000.000
注:TNF-α=腫瘤壞死因子α

Table 2Comparison of umbilical cord blood inflammatory cytokines levels in neonates with asthma and without asthma

組別例數白介素18白介素5白介素10白介素1白介素6TNF-α哮喘新生兒6618.2±108.3171.2±27.117.5±0.6143.0±16.4137.5±31.1143.7±21.3無哮喘新生兒54483.7±106.0117.0±25.219.0±4.6117.0±25.2109.6±25.6113.4±30.1t值2.9432.1452.2232.4632.4862.388P值0.0050.0360.0350.0170.0160.020
3 討論
哮喘是一種慢性氣道炎性疾病,炎癥在哮喘的發生、發展中起關鍵作用。哮喘的發病包括3個階段,即誘導期、速發相哮喘反應和遲發相哮喘反應,均有不同炎性細胞和特異性炎性細胞因子的參與[10-12]。免疫功能失調導致的氣道慢性炎癥是引發新生兒哮喘的主要病因,其可由病毒或細菌感染引起,主要病理表現為氣道黏膜水腫及嗜酸粒細胞、淋巴細胞和中性粒細胞浸潤,分泌物增多并含有大量炎性細胞和炎性因子,導致廣泛性的細小支氣管管腔狹窄或閉塞,最終導致新生兒哮喘。
白介素18、白介素5、白介素1、白介素6和TNF-α均是促進炎癥發展的炎性因子[13-14],上述炎性因子水平升高提示哮喘孕婦臍帶血炎性因子水平增加;白介素10是一種抑炎因子,其水平降低提示機體抑制炎癥的功能減弱[15-16]。有研究表明,哮喘患者白介素10水平降低可導致炎性因子過度表達,最終引發長期慢性氣道炎癥[17]。白介素10可有效抑制過敏原誘發的炎性反應,其是T輔助細胞的反應調節劑,可促進T細胞向抑制炎性反應的Th1輔助細胞轉化[18-19]。新生兒促炎因子水平增高而抑炎因子水平降低,表明哮喘孕婦臍帶血炎性因子水平升高。
本研究結果顯示,研究組新生兒臍帶血白介素18、白介素5、白介素1、白介素6、TNF-α水平高于對照組,臍帶血白介素10水平低于對照組;研究組新生兒哮喘發生率高于對照組;研究組哮喘新生兒臍帶血白介素18、白介素5、白介素1、白介素6、TNF-α水平高于無哮喘新生兒,臍帶血白介素10水平低于無哮喘新生兒。提示孕婦哮喘是新生兒哮喘的危險因素,哮喘孕婦臍帶血炎性因子水平升高可導致新生兒哮喘。覃萍等[20]研究表明,哮喘孕婦新生兒臍帶血白介素5水平高于健康孕婦(P<0.05)。
綜上所述,哮喘新生兒臍帶血炎性因子水平較高,早期檢測新生兒臍帶血炎性因子水平并采取有針對性的干預措施有助于減少新生兒哮喘的發生。但本研究僅為觀察性研究,不能完全明確臍帶血炎性因子水平升高在新生兒哮喘中的作用,有待進一步研究證實。
[1]HUANG L,BAO Y,XU Z,et al.Neonatal bilirubin levels and childhood asthma in the US Collaborative Perinatal Project,1959-1965[J].Am J Epidemiol,2013,178(12):1691-1697.
[3]GOKS?R E,ALM B,PETTERSSON R,et al.Early fish introduction and neonatal antibiotics affect the risk of asthma into school age[J].Pediatr Allergy Immunol,2013,24(4):339-344.
[4]KAUR B P,LAHEWALA S,ARORA S,et al.Asthma: Hospitalization Trends and Predictors of In-Hospital Mortality and Hospitalization Costs in the USA (2001—2010)[J].Int Arch Allergy Immunol,2015,168(2):71-78.
[5]NAKAFERO G,SANDERS R D,NGUYEN-VAN-TAM J S,et al.Association between benzodiazepine use and exacerbations and mortality in patients with asthma: a matched case-control and survival analysis using the United Kingdom Clinical Practice Research Datalink[J].Pharmacoepidemiol Drug Saf,2015,24(8):793-802.
[6]WATANABE T,FAJT M L,TRUDEAU J B,et al.Brain-Derived Neurotrophic Factor Expression in Asthma.Association with Severity and Type 2 Inflammatory Processes[J].Am J Respir Cell Mol Biol,2015,53(6):844-852.
[7]CHEN X J,ZHANG Y H,WANG D H,et al.Effects of body mass index and serum inflammatory cytokines on asthma control in children with asthma[J].Zhongguo Dang Dai Er Ke Za Zhi,2015,17(7):698-701.
[8]DING J,SU J,ZHANG L,et al.Crocetin Activates Foxp3 Through TIPE2 in Asthma-Associated Treg Cells[J].Cell Physiol Biochem,2015,37(6):2425-2433.
[9]OCZYPOK E A,MILUTINOVIC P S,ALCORN J F,et al.Pulmonary receptor for advanced glycation end-products promotes asthma pathogenesis through IL-33 and accumulation of group 2 innate lymphoid cells[J].J Allergy Clin Immunol,2015,136(3):747-756,e744.
[10]SUN Y,PENG I,WEBSTER J D,et al.Inhibition of the kinase ITK in a mouse model of asthma reduces cell death and fails to inhibit the inflammatory response[J].Sci Signal,2015,8(405):ra122.
[11]KIM H W,LIM C Y,KIM B Y,et al.So-Cheong-Ryong-Tang,a herbal medicine,modulates inflammatory cell infiltration and prevents airway remodeling via regulation of interleukin-17 and GM-CSF in allergic asthma in mice[J].Pharmacogn Mag,2014,10(Suppl 3):S506-511.
[12]KROEGEL C,BAKAKOS P.The inflammatory effector cell pattern in asthma and chronic obstructive pulmonary disease - what is it good for?[J].Respiration,2012,83(1):17-19.
[13]QI X,GURUNG P,MALIREDDI R K,et al.Critical role of caspase-8-mediated IL-1 signaling in promoting Th2 responses during asthma pathogenesis[J].Mucosal Immunol,2016.[Epub ahead of print].
[14]GRUBEK-JAWORSKA H,PAPLISKA M,HERMANOWICZ-SALAMON J,et al.IL-6 and IL-13 in induced sputum of COPD and asthma patients: correlation with respiratory tests[J].Respiration,2012,84(2):101-107.
[15]KAWANO H,KAYAMA H,NAKAMA T,et al.IL-10-producing lung interstitial macrophages prevent neutrophilic asthma[J].Int Immunol,2016.[Epub ahead of print].
[16]B?HM L,MAXEINER J,MEYER-MARTIN H,et al.IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma[J].J Immunol,2015,194(3):887-897.
[17]ZDRENGHEA M T,MAKRINIOTI H,MURESAN A,et al.The role of macrophage IL-10/innate IFN interplay during virus-induced asthma[J].Rev Med Virol,2015,25(1):33-49.
[18]TOUZOT M,CACOUB P,BODAGHI B,et al.IFN-α induces IL-10 production and tilt the balance between Th1 and Th17 in Beh?et disease[J].Autoimmun Rev,2015,14(5):370-375.
[19]KUMAR N P,MOIDEEN K,GEORGE P J,et al.Coincident diabetes mellitus modulates Th1-,Th2-,and Th17-cell responses in latent tuberculosis in an IL-10 and TGF-β dependent manner[J].Eur J Immunol,2016,46(2):390-399.
[20]覃萍,吳曙粵,王艷寧,等.哮喘產婦新生兒臍血IL-18、IL-5的檢測意義[J].吉林醫學,2016,37(8):1946-1947.
(本文編輯:李潔晨)
Change and Clinical Significance of Umbilical Cord Blood Inflammatory Cytokines Levels in Neonates with Asthma
MAChao,RENShao-min.
DepartmentofPediatrics,theAffiliatedHospitalofInnerMongoliaMedicalUniversity,Hohhot010050,China
RENShao-min,DepartmentofPediatrics,theAffiliatedHospitalofInnerMongoliaMedicalUniversity,Hohhot010050,China;E-mail:renshaomin722@126.com
Objective To analyze the change and clinical significance of umbilical cord blood inflammatory cytokines levels in neonates with asthma.MethodsA total of 60 pregnant women with asthma were selected as study group in the Affiliated Hospital of Inner Mongolia Medical University from 2012 to 2015,a total of 60 healthy pregnant women were selected as control group at the same time.Umbilical cord blood inflammatory cytokines(including IL-18,IL-5,IL-10,IL-1,IL-6 and TNF-α)levels were compared between the two groups,in neonates with asthma and without asthma,and incidence of asthma was compared between the two groups,too.ResultsUmbilical cord blood levels of IL-18,IL-5,IL-1,IL-6 and TNF-α of neonates of study group were statistically significantly higher than those of control group,while umbilical cord blood IL-10 level of neonates of study group was statistically significantly lower than that of control group(P<0.05).The incidence of asthma of neonates of study group was statistically significantly higher than that of control group(P<0.05).Of study group,umbilical cord blood levels of IL-18,IL-5,IL-1,IL-6 and TNF-α of neonates with asthma were statistically significantly higher than those of neonates without asthma,while umbilical cord blood IL-10 level of neonates with asthma was statistically significantly lower than that of neonates without asthma(P<0.05).ConclusionUmbilical cord blood inflammatory cytokines levels of neonates with asthma are significantly elevated,early detection of umbilical cord blood inflammatory cytokines levels of neonates and carrying out targeted intervention measures are helpful to reduce the incidence of asthma.
Asthma;Infant,newborn;Fetal blood;Inflammatory factor
內蒙古自治區自然科學基金項目(2012MS1120)
010050內蒙古自治區呼和浩特市,內蒙古醫科大學附屬醫院兒科
任少敏,010050內蒙古自治區呼和浩特市,內蒙古醫科大學附屬醫院兒科;E-mail:renshaomin722@126.com
R 562.25
B
10.3969/j.issn.1008-5971.2016.09.021
2016-05-05;
2016-08-20)

