姜黃素對(duì)TNF-α誘導(dǎo)人結(jié)直腸癌細(xì)胞上皮間充質(zhì)轉(zhuǎn)化、遷移的影響
李倩,徐春燕,彭鵬,覃蒙斌,李素艷,黃杰安
(廣西醫(yī)科大學(xué)第一附屬醫(yī)院,南寧530021)
?
·論著·
姜黃素對(duì)TNF-α誘導(dǎo)人結(jié)直腸癌細(xì)胞上皮間充質(zhì)轉(zhuǎn)化、遷移的影響
李倩,徐春燕,彭鵬,覃蒙斌,李素艷,黃杰安
(廣西醫(yī)科大學(xué)第一附屬醫(yī)院,南寧530021)
目的觀察姜黃素對(duì)腫瘤壞死因子-α(TNF-α)誘導(dǎo)結(jié)直腸癌細(xì)胞上皮間充質(zhì)轉(zhuǎn)化(EMT)的影響,并探討其可能的機(jī)制。方法 將人結(jié)直腸癌HCT116細(xì)胞分為3組,A、B組分別以TNF-α 20 ng/mL、TNF-α 20 ng/mL+姜黃素25 μmol/L干預(yù)48 h,C組不干預(yù)。采用相差顯微鏡觀察細(xì)胞形態(tài)變化,劃痕實(shí)驗(yàn)觀察細(xì)胞遷移能力,Western blot法檢測(cè)EMT標(biāo)記物E-鈣黏蛋白(E-cadherin)、波形蛋白(Vimentin)及NF-κB信號(hào)通路的p65蛋白。結(jié)果 A組細(xì)胞多呈具有極性的紡錘狀、長(zhǎng)梭形,排列疏松,大部分出現(xiàn)細(xì)長(zhǎng)偽足;B組細(xì)長(zhǎng)偽足細(xì)胞較A組少見,凋落細(xì)胞明顯多于A組;C組細(xì)胞大多數(shù)為多邊形,細(xì)胞間存在一定的間隙,少量細(xì)胞出現(xiàn)短粗偽足。A、B、C組細(xì)胞遷移距離分別為(319.84±20.93)、(90.70±7.25)、(144.07±11.31)μm,P均<0.05。與C組比較,A組細(xì)胞E-cadherin表達(dá)減少,Vimentin、p65蛋白表達(dá)增加(P均<0.05或0.01);而B組較A組E-cadherin表達(dá)增加,Vimentin、p65蛋白表達(dá)減少(P均<0.05或0.01)。結(jié)論 姜黃素可能通過NF-κB信號(hào)通路來抑制TNF-α誘導(dǎo)結(jié)直腸癌HCT116細(xì)胞的EMT發(fā)生、侵襲和轉(zhuǎn)移。
結(jié)直腸癌;HCT116細(xì)胞;姜黃素;上皮間質(zhì)轉(zhuǎn)化;核轉(zhuǎn)錄因子κB;腫瘤壞死因子
據(jù)統(tǒng)計(jì),我國2015年最常見的惡性腫瘤中,結(jié)直腸癌在男性中排第5位、女性中排第4位[1]。腫瘤患者絕大部分最終死于腫瘤轉(zhuǎn)移,所以對(duì)腫瘤轉(zhuǎn)移的治療尤為重要。上皮間質(zhì)轉(zhuǎn)化(EMT)是指在一定的生理和病理情況下,上皮細(xì)胞在形態(tài)學(xué)上向間質(zhì)細(xì)胞表型轉(zhuǎn)變的現(xiàn)象;EMT時(shí)腫瘤細(xì)胞間排列疏松、大部分出現(xiàn)細(xì)長(zhǎng)偽足,并出現(xiàn)E-鈣黏蛋白(E-cadherin)表達(dá)減少、波形蛋白(Vimentin)表達(dá)增加。這種轉(zhuǎn)變使腫瘤細(xì)胞擺脫細(xì)胞間的黏附作用,獲得遷移、侵襲能力[2,3]。腫瘤壞死因子-α(TNF-α)能殺傷或抑制腫瘤細(xì)胞,但在一定濃度下促進(jìn)腫瘤細(xì)胞發(fā)生EMT[4]。本課題組前期研究表明,20 ng/mL的TNF-α可激活NF-κB通路釋放p65亞基,促進(jìn)人結(jié)直腸癌細(xì)胞HCT116的EMT發(fā)生、侵襲和轉(zhuǎn)移[5]。因此,尋找抑制或逆轉(zhuǎn)這一過程的藥物很有應(yīng)用前景。姜黃素是一種從姜科植物姜黃中提取的天然多酚類物質(zhì),具有多種生物學(xué)作用[6]。文獻(xiàn)[7,8]報(bào)道,姜黃素通過阻斷細(xì)胞周期、抑制炎癥反應(yīng)、抗氧化作用及改變腫瘤微環(huán)境等多種方式促進(jìn)結(jié)直腸癌細(xì)胞的凋亡。但是,姜黃素在結(jié)直腸癌中的抗癌作用與EMT之間的調(diào)節(jié)關(guān)系仍不明確。2015年2月~2016年1月,我們觀察了姜黃素對(duì)HCT116細(xì)胞EMT及遷移的影響,并探討其機(jī)制。
1 材料與方法
1.1材料HCT116細(xì)胞購自中國科學(xué)院細(xì)胞庫;TNF-α、姜黃素(>99.0%)均購自美國Sigma-Aldrich公司;兔抗人p65和E-cadherin單克隆抗體均購自美國Cell Signaling Technology公司;兔抗人Vimentin購自美國Proteintech公司;鼠抗人GAPDH購自美國Proteintech公司;胎牛血清購自美國ExCell公司;DMEM高糖培養(yǎng)基購自美國Gibco公司。
1.2實(shí)驗(yàn)方法
1.2.1HCT116細(xì)胞培養(yǎng)將HCT116細(xì)胞用含10%胎牛血清的培養(yǎng)液于5% CO2、37 ℃的細(xì)胞培養(yǎng)箱中常規(guī)培養(yǎng),待細(xì)胞在培養(yǎng)瓶中長(zhǎng)至約鋪滿90%瓶底面積時(shí)傳代。
1.2.2HCT116細(xì)胞EMT形態(tài)觀察采用相差顯微鏡。取對(duì)數(shù)生長(zhǎng)期HCT116細(xì)胞,消化后制成細(xì)胞懸液,以2×104/孔接種至6孔板。細(xì)胞貼壁后分為3組,A、B組分別以TNF-α 20 ng/mL、TNF-α 20 ng/mL+姜黃素25 μmol/L干預(yù)48 h,C組常規(guī)培養(yǎng)不干預(yù)。每組分別設(shè)3個(gè)復(fù)孔,相差顯微鏡觀察細(xì)胞形態(tài)變化并拍照。
1.2.3HCT116細(xì)胞遷移能力觀察采用細(xì)胞劃痕實(shí)驗(yàn)。取對(duì)數(shù)生長(zhǎng)期HCT116細(xì)胞,以3×105/孔接種于6孔板。細(xì)胞貼壁后分組與干預(yù)同1.2.2,每組設(shè)3個(gè)平行板。干預(yù)48 h后,用高壓消毒的200 μL移液器槍頭垂直劃線;用無菌PBS輕輕沖洗細(xì)胞3次,洗脫劃下的細(xì)胞。加入無血清DMEM高糖培養(yǎng)基,于37 ℃孵育箱常規(guī)培養(yǎng)。分別在劃線后0、48 h使用倒置顯微鏡拍照,以遷移距離評(píng)價(jià)細(xì)胞遷移能力。遷移距離=0 h劃痕距離-48 h劃痕距離。
1.2.4HCT116細(xì)胞E-cadherin、Vimentin、p65蛋白檢測(cè)采用Western blot法。取對(duì)數(shù)生長(zhǎng)期HCT116細(xì)胞分別接種于培養(yǎng)瓶中,細(xì)胞貼壁后分組與干預(yù)同1.2.2。干預(yù)48 h后收集各組細(xì)胞,加裂解液裂解細(xì)胞并提取總蛋白;核酸檢測(cè)儀測(cè)定蛋白質(zhì)濃度后,各組以每孔40 μg蛋白上樣。濃縮膠上電泳電壓為60 V,分離膠上電泳電壓為100 V,電泳3 h。使用硝酸纖維素膜轉(zhuǎn)膜,用5%脫脂牛奶封閉后分別加E-cadherin蛋白抗體、Vimentin蛋白抗體、p65蛋白抗體和GAPDH抗體4 ℃孵育過夜。以Tris-PBS溶液洗膜5 min×3次,室溫下避光孵育二抗1 h;用Tris-PBS溶液洗膜5 min×3次,用Odyssey雙色紅外熒光掃膜成像。以目的蛋白與GAPDH灰度比值表示各目的蛋白的相對(duì)表達(dá)量。

2 結(jié)果
2.1各組細(xì)胞EMT形態(tài)變化A組細(xì)胞多呈具有極性的紡錘狀、長(zhǎng)梭形,排列疏松,大部分出現(xiàn)細(xì)長(zhǎng)偽足;B組細(xì)胞呈現(xiàn)過渡形態(tài),紡錘狀、長(zhǎng)梭形細(xì)胞與多邊形細(xì)胞堆疊,細(xì)長(zhǎng)偽足細(xì)胞較A組少見,凋落細(xì)胞明顯多于A組;C組細(xì)胞大多數(shù)為多邊形,細(xì)胞間存在一定的間隙,少量細(xì)胞出現(xiàn)短粗偽足。
2.2各組細(xì)胞遷移能力比較 A、B、C組細(xì)胞遷移距離分別為(319.84±20.93)、(90.70±7.25)、(144.07±11.31)μm,三組比較P均<0.05。
2.3各組細(xì)胞E-cadherin、Vimentin、p65表達(dá)比較見表1。
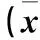
表1 各組細(xì)胞E-cadherin、Vimentin、p65相對(duì)表達(dá)量比較±s)
注:與C組相比,*P<0.05,**P<0.01;與A組相比,△P<0.05,△△P<0.01。
3 討論
EMT最早于1982年由Greenburg等[9]在晶狀體上皮細(xì)胞的培養(yǎng)中發(fā)現(xiàn)。EMT的過程中,上皮細(xì)胞形態(tài)具有極性,胞體變得細(xì)長(zhǎng),呈紡錘狀,長(zhǎng)出細(xì)長(zhǎng)游離的絲狀偽足;細(xì)胞排列疏松,失去細(xì)胞間的黏附力。因此,上皮細(xì)胞能逐漸脫離上皮,侵入細(xì)胞間,最終向遠(yuǎn)處組織轉(zhuǎn)移。研究顯示,大劑量的TNF-α能通過抑制腫瘤血管起到抗腫瘤作用,而低劑量作用下可以誘導(dǎo)EMT促進(jìn)多種腫瘤細(xì)胞的生長(zhǎng)[4,10]。另外,細(xì)胞間黏附因子E-cadherin表達(dá)下調(diào)被認(rèn)為是EMT的標(biāo)志[11],以角蛋白為主的細(xì)胞骨架變?yōu)橐訴imentin為主的細(xì)胞骨架。本實(shí)驗(yàn)結(jié)果表明,在TNF-α作用下,HCT116細(xì)胞呈現(xiàn)EMT形態(tài)學(xué)改變,其E-cadherin表達(dá)減少、Vimentin表達(dá)增加,與上述研究結(jié)果一致。
研究發(fā)現(xiàn),EMT的發(fā)生與STAT、AKT、NF-κB等多條信號(hào)通路有關(guān)[2,12,13]。Yan等[14]發(fā)現(xiàn),在結(jié)直腸癌細(xì)胞中,穩(wěn)定過表達(dá)整合素連接激酶(ILK)能發(fā)生EMT;利用NF-κB小干擾RNA作用后,顯著增加過表達(dá)ILK細(xì)胞株的E-cadherin水平。這表明結(jié)直腸癌細(xì)胞可以通過NF-κB信號(hào)通路誘導(dǎo)EMT。TNF-α可以通過激活p38絲裂原活化蛋白激酶(MAPK)[15]和酪氨酸激酶/STAT信號(hào)通路促進(jìn)結(jié)直腸癌發(fā)生EMT,也能通過活化NF-κB信號(hào)通路調(diào)節(jié)蛋白誘導(dǎo)EMT的發(fā)生。
研究認(rèn)為,姜黃素可通過調(diào)節(jié)抗氧化反應(yīng)、免疫反應(yīng)、凋亡、細(xì)胞周期、血管生成等相關(guān)蛋白而發(fā)揮其抗腫瘤作用[6]。有研究顯示,姜黃素能在乳腺癌細(xì)胞中通過NF-κB信號(hào)通路抑制EMT的發(fā)生,從而抑制腫瘤遠(yuǎn)處轉(zhuǎn)移。但是,姜黃素在結(jié)直腸癌中是否通過NF-κB信號(hào)通路抑制EMT發(fā)生從而抗腫瘤遠(yuǎn)處轉(zhuǎn)移尚未見報(bào)道。本研究結(jié)果顯示,加入TNF-α的A組細(xì)胞間排列疏松、大部分出現(xiàn)細(xì)長(zhǎng)偽足,腫瘤細(xì)胞間的黏附減弱而與細(xì)胞外基質(zhì)黏附增強(qiáng),本應(yīng)緊密排列的上皮樣結(jié)構(gòu)逐漸向間質(zhì)細(xì)胞形態(tài)轉(zhuǎn)變;而加入姜黃素的B組細(xì)胞排列較A組緊密,長(zhǎng)偽足細(xì)胞明顯減少。并且,A組細(xì)胞較C組遷移距離增加,而B組較A組遷移距離縮短;A組細(xì)胞E-cadherin表達(dá)下調(diào),Vimentin和NF-κB信號(hào)通路p65蛋白表達(dá)增加;而加入了姜黃素作用的B組細(xì)胞E-cadherin表達(dá)減少,Vimentin和NF-κB信號(hào)通路p65蛋白表達(dá)減少。因此,我們認(rèn)為,姜黃素可能通過抑制NF-κB信號(hào)通路的p65蛋白表達(dá)來抑制EMT的發(fā)生,進(jìn)而抑制結(jié)直腸癌細(xì)胞的運(yùn)動(dòng)遷移,從而起到抗腫瘤作用,這為姜黃素治療癌癥提供了又一理論基礎(chǔ)。
[1] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China,2015[J]. CA, 2016,66(2):115-1132.
[2] Lee MH, Kachroo P, Pagano PC, et al. Combination treatment with apricoxib and IL-27 enhances inhibition of epithelial-mesenchymal transition in human lung cancer cells through a STAT1 dominant pathway[J]. J Cancer Sci Ther, 2014,6(11):468-477.
[3] Savagner P. The epithelial-mesenchymal transition(EMT) phenomenon[J]. ESMO, 2010,21(7):89-92.
[4] Balkwill F. Tumor necrosis factor or tumor promoting factor[J]. Cyto Grow Fact Rev, 2002,13(2):135-141.
[5] 劉寶玉,黃杰安,劉詩權(quán),等.NF-κB對(duì)人結(jié)直腸癌細(xì)胞上皮間質(zhì)轉(zhuǎn)化及侵襲轉(zhuǎn)移的影響[J]. 世界華人消化雜志,2014,22(23):3403-3409.
[6] Kumar G, Mittal S, Sak K, et al. Molecular mechanisms underlying chemopreventive potential of curcumin: current challenges and future perspectives[J]. Life Sci, 2016,148(3):313-328.
[7] Lim TG, Lee SY, Huang Z, et al. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2[J]. Cancer Prev Res, 2014,7(4):466-474.
[8] Buhrmann C, Kraehe P, Lueders C, et al. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: potential role of EMT[J]. PLoS One, 2014,9(9):107514.
[9] Greenburg G,Hay DE. Epithelia duspended in collagen gels can lose characteristics of migrating mesenchymal cellspolarity and express[J]. J Cell Biol, 1982,95(1):333-939.
[10] Bigatto V, De Bacco F, Casanova E, et al. TNF-alpha promotes invasive growth through the MET signaling pathway[J]. Mol oncol, 2015,9(2):377-388.
[11] Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology[J]. Pathology, 2007,39(3):305-318.
[12] Li W, Cao L, Han L, et al. Superoxide dismutase promotes the epithelial-mesenchymal transition of pancreatic cancer cells via activation of the H2O2/ERK/NF-kappaB axis[J]. Int J Oncol, 2015,46(6):2613-2620.
[13] Wang H, Wang HS, Zhou BH, et al. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer [J]. PLoS One, 2013,8(2):56664.
[14] Yan Z, Yin H, Wang R, et al. Overexpression of integrin-linked kinase (ILK) promotes migration and invasion of colorectal cancer cells by inducing epithelial-mesenchymal transition via NF-kappaB signaling[J]. Acta Histochem, 2014,116(3):527-533.
[15] Bates RC, Mercurio AM. Tumor necrosis factor alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids[J]. Mol Biol Cell, 2003,14(5):1790-1800.
Effect of curcumin on epithelial-mesenchymal transition and migration of colorectal cancer cells induced by TNF-α
LIQian,XUChunyan,PENGPeng,QINMengbin,LISuyan,HUANGJiean
(TheFirstAffiliatedHospitalofGuangxiMedicalUniversity,Nanning530021,China)
ObjectiveTo investigate the effect of curcumin on tumor necrosis factor-α (TNF-α)-induced epithelial-mesenchymal transition (EMT) of colorectal cancer HCT116 cells and the possible mechanism. MethodsThe human colorectal cancer HCT116 cells were divided into three groups. Group A and group B were treated with 20 ng/mL TNF-α, 20 ng/mL TNF-α + 25 μmol/L curcumin for 48 h, respectively, and group C with routine culture. Using phase contrast microscope to observe the changes in cell morphology, cell scratch experiments to observed cell migration in each group. Western blotting was used to detect the expression levels of EMT markers E-cadherin, Vimentin and NF-κB signaling pathway-related P65 protein. Results Cells of group A were mostly spindle-shaped, fusiform and loosely arranged and most appeared elongated pseudopodia; in the group B, the slender pseudopodia cells were less, and apoptotic cells were more than those in the group A; most of cells in the group C were polygon, there was a little space between cells, and a few cells appeared stubby pseudopodia. The cell migration distance of groups A, B and C were (319.84±20.93) μm, (90.70±7.25) μm, (144.07±11.31) μm, respectively, and significant difference was found between these three groups (allP<0.05). Compared with group C, the expression of E-cadherin in cells of group A was decreased, however, the expression of Vimentin and p65 protein was increased; and compared with group A, the expression of E-cadherin in cells of group B was increased, the expression of Vimentin and p65 protein was reduced. ConclusionCurcumin inhibited the occurrence, development and metastasis of TNF-α-induced EMT in HCT116 colorectal cancer cells probably through NF-κB signaling pathway.
colorectal carcinoma; HCT116 cells; curcumin; epithelial-mesenchymal transition; nuclear transcription factor-κB; tumor necrosis factor
國家自然科學(xué)基金資助項(xiàng)目(81260365);廣西中醫(yī)藥民族醫(yī)藥自籌經(jīng)費(fèi)科研課題項(xiàng)目(GZZC14-57)。
李倩(1990-),女,碩士研究生,主要研究方向?yàn)橄滥[瘤的分子機(jī)制。E-mail: qiandeyu19@163.com
簡(jiǎn)介:黃杰安(1965-),男,教授,主任醫(yī)師,博士生導(dǎo)師,主要研究方向?yàn)橄滥[瘤的分子機(jī)制。E-mail: 1404991727@qq.com
10.3969/j.issn.1002-266X.2016.34.001
735.3
A
1002-266X(2016)34-0001-03
2016-03-28)

