Shh信號通路阻斷對人結腸癌細胞HT-29增殖、侵襲的影響及機制探討
梁新軍,魏少忠
(湖北省腫瘤醫院,武漢430079)
?
Shh信號通路阻斷對人結腸癌細胞HT-29增殖、侵襲的影響及機制探討
梁新軍,魏少忠
(湖北省腫瘤醫院,武漢430079)
目的觀察阻斷Sonic Hedgehog(Shh)信號通路對人結腸癌細胞HT-29增殖和侵襲的影響,并探討其機制。方法取對數生長期HT-29細胞分為A、B、C、D組,分別置于含0、5、10、20 μmol/L Shh信號通路特異性抑制劑Cyclopamine的培養基中培養。采用MTT法檢測各組干預24、48、72 h時細胞增殖抑制率,采用Transwell法檢測各組干預48 h時體外侵襲能力,采用實時熒光定量PCR法檢測各組細胞Shh、下游轉錄因子GLI1 mRNA。結果干預24、48、72 h時各組細胞增殖抑制率為B組
結腸癌;Shh信號通路;下游轉錄因子;細胞增殖;細胞侵襲
結腸癌是臨床常見的惡性腫瘤之一,發病率已躍居全球惡性腫瘤的第3位,其發病機制目前尚不明確[1]。基因突變和表觀遺傳學改變等參與了結腸癌的發生、發展過程[2]。Sonic Hedgehog(Shh)信號通路是調控胚胎發育及細胞分化的重要通路,GLI1是其下游轉錄因子。研究發現,Shh信號通路在肺癌、乳腺癌、胃癌、結腸癌等多種惡性腫瘤中呈激活狀態,且與腫瘤的惡性程度和患者預后密切相關[3~5]。目前Shh信號通路在結腸癌中的具體作用機制的研究報道較少。2015年1~12月,我們觀察了阻斷Shh信號通路對人結腸癌細胞HT-29增殖和侵襲的影響,并探討其在結腸癌發生、發展中的意義。現報告如下。
1 材料與方法
1.1材料HT-29購自美國ATCC公司,置于含10%胎牛血清的RPMI 1640培養基中,于37 ℃、5%CO2、飽和濕度的培養箱內培養。胎牛血清、RPMI 1640培養基及TRIzol為美國Gibco公司產品;胰酶、DMSO、MTT、Shh信號通路特異性抑制劑Cyclopamine等購自Sigma公司;SYBR Premix EX Taq試劑盒購自Invitrogen公司;引物由上海吉瑪公司合成;Transwell小室購自Corning公司;Bio-Rad iQ5實時熒光定量PCR儀購自Bio-Rad公司;Multiskan MK3全自動酶標儀購自Thermo公司。
1.2細胞分組及處理取對數生長期HT-29細胞,胰酶消化、重懸后分為A、B、C、D組,分別置于含0、5、10、20 μmol/L Cyclopamine的培養基中培養,每組6個復孔,均置于37 ℃、5% CO2、飽和濕度的培養箱內培養。
1.3HT-29增殖情況觀察采用MTT法。分別于干預24、48、72 h取對數生長期各組細胞,調整細胞密度5×104/mL。每孔200 μL接種于96孔板,每組6個復孔。培養24 h,加入20 μL的MTT(5 g/L)行MTT檢測,所有操作均嚴格按照使用說明書進行。采用酶標儀檢測各組490 nm波長處的吸光度值,計算增殖抑制率。細胞增殖抑制率=(對照組吸光度值-實驗組吸光度值)/對照組吸光度值×100%。實驗重復3次。
1.4HT-29侵襲能力觀察采用Transwell法。干預48 h時取對數生長期各組細胞,1×105/mL培養在無血清的PRMI 1640培養基中,置于Transwell小室上室,下室每孔加入0.5 mL含10%胎牛血清的PRMI 1640培養基。置于37 ℃、5% CO2、飽和濕度的培養箱中,培養24 h。拭去上室表面剩余細胞,將遷移到下室的細胞用多聚甲醛固定30 min,Giemsa染色15 min;顯微鏡下計數,以穿膜細胞數表示細胞的侵襲能力。實驗重復3次。
1.5細胞Shh、GLI1 mRNA檢測采用實時熒光定量PCR法。干預48 h時取對數生長期各組細胞,照TRIzol試劑盒說明提取總RNA,逆轉錄合成cDNA。按照SYBR試劑盒說明進行實時熒光定量PCR,對Shh、GLI1的mRNA進行定量檢測。引物采用Primer 5.0軟件設計,具體如下:Shh上游引物5′-CCAACGTAGCCGAGAAGACC-3′,下游引物5′-TCC-CGTGTTTCCTCATCCT-3′;Gli1上游引物5′-TGAGGTGGGCAGGTTAGGA-3′,下游引物5′-CAGAGGGAGATGGGGTGTTTT-3′。內參GAPDH上游引物5′-TGTCCCCACCCCCAATGTATC-3′,下游引物5′-CTCCGATGC CTGCTTCACACCTT-3′。反應條件:94 ℃預變性5 min;94 ℃、30 s,58 ℃、30 s,72 ℃、30 s,共35個循環。以GAPDH為內參,采用2-ΔΔCt法計算目的基因的相對表達量。
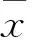
2 結果
2.1各組細胞增殖抑制率比較見表1。

表1 干預24、48、72 h時各組細胞增殖抑制率比較
注:與B組同時點比較,#P<0.05;與C組同時點比較,*P<0.05;與本組前一時點比較,△P<0.05。
2.2各組穿膜細胞數比較干預48 h時,A、B、C、D組的穿膜細胞數分別為(55.670±6.506)、(35.330±7.371)、(28.330±4.041)、(23.330±2.309)個。B、C、D組穿膜細胞數均低于A組(P均<0.05),但B、C、D組間比較差異無統計學意義。
2.3各組Shh、GLI1 mRNA表達比較各組Shh、GLI1 mRNA的相對表達量均為A組>B組>C組>D組,P均<0.05。見表2。

表2 各組HT-29組織Shh、GLI1 mRNA相對表達量比較
注:與A組比較,*P<0.05;與B組比較,#P<0.05;與C組比較,ΔP<0.05。
3 討論
目前認為結腸癌的發生是多因素、多步驟的復雜過程,基因突變和表觀遺傳學改變等均參與了結腸癌的侵襲與轉移過程,但其確切機制尚不明確[6~8]。國內外學者已發現多個與結腸癌轉移相關的基因,如VEGF、MMP-9等,并針對這些因子采取了包括藥物、基因轉染等多種方法進行調控,雖能取得部分抑制轉移的效果,但結果尚不滿意[9~11]。
Shh信號轉導通路不僅在胚胎發育過程中發揮重要作用,也參與了組織修復和細胞再生以及腫瘤發生和發展的過程。目前認為,Shh信號轉導通路主要由Hh信號肽、跨膜受體以及下游轉錄分子組成,通過復雜的信號轉導過程將上游信號傳遞到核內,進一步促進下游基因的表達而參與細胞的各項調控過程[12,13]。研究表明,Shh信號轉導通路的關鍵信號分子主要有Shh、跨膜蛋白受體Ptch、Smo及下游的轉錄因子Gli等。在Shh信號被激活后,Shh與跨膜蛋白受體Ptch(Ptch1、Ptch2)相結合,解除了其對Smo的抑制作用,繼而傳遞信號至下游的Gli(Gli1、Gli2、Gli3),Gli蛋白進入核內后進一步激活下游NF-κB、Bmi-1等因子的表達,從而參與調控細胞的增殖與凋亡過程。Shh信號可能通過多種機制參與調控惡性腫瘤細胞的增殖與侵襲[14,15]。Sharma等[16]研究發現,Shh信號可能與PI3K/Akt/mTOR信號共同參與了胰腺癌干細胞增殖和分化的調控過程,繼而參與了胰腺癌的侵襲與轉移。Giakoustidis等[17]研究認為,Shh信號、WNT/β-catenin信號及Notch信號等可能共同參與了肝癌侵襲與轉移的過程。本研究前期研究發現,結腸癌中Shh信號通路呈異常激活狀態,本研究發現,干預24、48、72 h時各組細胞增殖抑制率均為B組
[1] Siegel R, Desantis C, Jemal A. Colorectal cancer statistics[J]. CA Cancer J Clin, 2014,64(2):104-117.
[2] Storm EE, Durinck S, de Sousa Melo F, et al. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function[J]. Nature, 2016,529(7584):97-100.
[3] Bora-Singhal N, Perumal D, Nguyen J, et al. Gli1-mediated regulation of sox2 facilitates self-renewal of stem-like cells and confers resistance to EGFR inhibitors in non-small cell lung cancer[J]. Neoplasia, 2015,17(7):538-551.
[4] Duan ZH, Wang HC, Zhao DM, et al. Cooperatively transcriptional and epigenetic regulation of sonic hedgehog overexpression drives malignant potential of breast cancer[J]. Cancer Sci, 2015,106(8):1084-1091.
[5] Xu M, Gong A, Yang H, et al. Sonic hedgehog-glioma associated oncogene homolog 1 signaling enhances drug resistance in CD44(+)/Musashi-1(+) gastric cancer stem cells[J]. Cancer Lett, 2015,369(1):124-133.
[6] Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage Ⅲ colon cancer: final results of the NO16968 randomized controlled phase Ⅲ trial[J]. J Clin Oncol, 2015,33(32):3733-3740.
[7] de Ridder JA, van der Stok EP, Mekenkamp LJ, et al. Management of liver metastases in colorectal cancer patients: A retrospective case-control study of systemic therapy versus liver resection[J]. Eur J Cancer, 2016(59):13-21.
[8] Wada Y, Takami Y, Tateishi M, et al. Efficacy of surgical treatment using microwave coagulo-necrotic therapy for unresectable multiplecolorectal liver metastases[J]. Onco Targets Ther, 2016(9):937-943.
[9] Artemov A, Aliper A, Korzinkin M, et al. A method for predicting target drug efficiency in cancer based on the analysis of signaling pathway activation[J]. Oncotarget, 2015,6(30):29347-29356.
[10] Kim DH, Sung B, Kang YJ, et al. Sulforaphane inhibits hypoxia-induced HIF-1α and VEGF expression and migration of human colon cancer cells[J]. Int J Oncol, 2015,47(6):2226-2232.
[11] Goos JA, Coupé VM, van de Wiel MA, et al. A prognostic classifier for patients with colorectal cancer liver metastasis, based on AURKA, PTGS2 and MMP9[J]. Oncotarget, 2016,7(2):2123-2134.
[12] Jung IH, Jung DE, Park YN, et al. Aberrant Hedgehog ligands induce progressive pancreatic fibrosis by paracrine activation of myofibroblasts and ductular cells in transgenic zebrafish[J]. PLoS One, 2011,6(12):27941.
[13] Ransohoff KJ, Sarin KY, Tang JY. Smoothened Inhibitors in Sonic Hedgehog Subgroup Medulloblastoma[J]. J Clin Oncol, 2015,33(24):2692-2694.
[14] Wang ZC, Gao J, Zi SM, et al. Aberrant expression of sonic hedgehog pathway in colon cancer and melanosis coli[J]. J Dig Dis, 2013, 14(8):417-424.
[15] Xu X, Liu H, Zhang H, et al. Sonic hedgehog-GLI family zinc finger 1 signaling pathway promotes the growth and migration of pancreatic cancer cells by regulating the transcription of eukaryotic translation initiation factor 5A2[J]. Pancreas,2015,44(8):1252-1258.
[16] Sharma N, Nanta R, Sharma J, et al. PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreaticcancer stem cell characteristics and tumor growth[J]. Oncotarget, 2015,6(31):32039-32060.
[17] Giakoustidis A, Giakoustidis D, Mudan S, et al. Molecular signalling in hepatocellular carcinoma: Role of and crosstalk among WNT/β-catenin, Sonic Hedgehog, Notch and Dickkopf-1[J]. Can J Gastro Hepat, 2015,29(4):209-217.
Effects of blocking Shh signaling pathway on proliferation and invasion of colon cancer cell line HT-29
LIANGXinjun,WEIShaozhong
(HubeiCancerHospital,Wuhan430079,China)
ObjectiveTo observe the effects of blocking Sonic Hedgehog (Shh) signaling pathway on the proliferation and invasion of colon cancer cell line HT-29 and to investigate the mechanism. MethodsHT-29 cells in the logarithmic phase were divided into groups A, B, C, and D and were cultured in the culture medium containing 0, 5, 10 and 20 mol/L Shh signaling pathway specific inhibitor Cyclopamine, respectively. The inhibitory rate of cell proliferation was detected by MTT method at 24, 48 and 72 h after the treatment of Cyclopamine. Transwell method was used to detect the invasive ability of each group at 48 h. The Shh and downstream transcription factor GLI1 mRNA in HT-29 cells was detected by real-time fluorescence quantitative PCR. ResultsThe proliferation inhibition rate at 24 h, 48 h and 72 h: group B
colon carcinoma; Sonic Hedgehog signaling pathway; downstream transcription factor; cell proliferation; cell invasion
湖北省武漢市科技局科技計劃項目(2013062301010813);湖北省武漢市晨光計劃(2015070404010204)。
梁新軍(1976-),男,博士,副主任醫師,主要研究方向為腫瘤免疫。E-mail: doctorlxj@163.com。
10.3969/j.issn.1002-266X.2016.18.004
R735.3
A
1002-266X(2016)18-0012-03
2016-01-11)

