Estimation of the Ballistic Effectiveness of 3,4- and 3,5-Dinitro-1-(trinitromethyl)-1H-Pyrazoles as Oxidizers for Composite Solid Propellants
LEMPERT David B. , DALINGER Igor L., SHU Yuan-jie, KAZAKOV Anatolii I., SHEREMETEV Aleksei B.
(1.Institute of Problems of Chemical Physics, Russian Academy of Sciences(IPCP RAS), Moscow 142432, Russia; 2.Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow 119991, Russia; 3.Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
?
Estimation of the Ballistic Effectiveness of 3,4- and 3,5-Dinitro-1-(trinitromethyl)-1H-Pyrazoles as Oxidizers for Composite Solid Propellants
LEMPERT David B.1, DALINGER Igor L.2, SHU Yuan-jie3, KAZAKOV Anatolii I.1, SHEREMETEV Aleksei B.2
(1.Institute of Problems of Chemical Physics, Russian Academy of Sciences(IPCP RAS), Moscow 142432, Russia; 2.Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow 119991, Russia; 3.Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
Abstract:The experimental values of the enthalpy of formation of two isomeric 3,4- and 3,5-dinitro-1-(trinitromethyl)-1H-pyrazoles have been obtained (261.5±5.0 and 246.4±6.7 kJ/mol for crystalline 3,4- and 3,5-dinitro-1-(trinitromethyl)-1H-pyrazoles, respectively). The ballistic effectiveness of these potential oxidizers in composite solid propellants was studied. It is shown that these two oxidizers may be successfully applied in metal-free compositions or with a small content of metal. For the bottom stage 3,4-dinitro-1-(trinitromethyl)-1H-pyrazole is a bit better than 3,5-dinitro-1-( trinitromethyl)-1H-pyrazole, for the upper stage the both oxidizers show the equal ballistic parameters. These oxidizers allow to create metal-free solid composite propellants with the binder percentage not lower than 19% (volume fraction), with Ispequal to 256.5-257.0s at density equal to 1.72-1.74g/cm3.
Keywords:composite solid propellants; high-enthalpy oxidizer; energetic parameters estimation; specific impulse; ballistic effectiveness
Introduction
Currently, there is a considerable interest in the synthesis and chemistry of polynitropyrazoles[1-2]. The thermal stable compounds are primarily interested because of their energetic properties as components of explosives and propellants. The design and synthesis of new energetic pyrazoles with performance properties comparable or superior to RDX but with less sensitivity toward certain stimuli have been the focus of recent studies in our and other research groups worldwide[3-7].
In the decade, novel green energetic highly over-oxidized high-enthalpy polynitrogen compounds have drawn the interest of the scientific community due to their unique potential for solid rocket propellant formulations and gas generant compositions. During the time, the solid composite propellants effort at IPCP RAS has been directed toward using the novel materials to increase energetic characteristics[8-14].
In this study, we introduce two new energetic components-3,4-dinitro-1-(trinitromethyl)-1H-pyrazole and 3,5-dinitro-1-(trinitromethyl)-1H-pyrazole in the composite solid propellants. The experimental values of the enthalpy of formation of these isomeric have been obtained. The ballistic effectiveness of these potential oxidizers in solid composite propellants was studied.
1Calculation of the Enthalpy and the Principle of Ballistic Efficiency
Recently, two isomeric polynitropyrazoles (C4HN7O10), 3,4-dinitro-1-(trinitromethyl)-1H-pyrazoles (I) and 3,5-dinitro-1-(trinitromethyl)-1H-pyrazoles (II), with high oxygen balanceα=1.18 (α= O/(2C+0.5H) have been described[15]. The compounds exhibit excellent X-ray densities (1.906g/cm3for I and 1.937g/cm3for II). The estimated values of the standard enthalpy of formation (ΔH°f) in the gas phase are equal to 832.7kJ/kg and 816.7kJ/kg, respectively (calculated by PM3 method). These data allow to hope that compound I and II may become rather effective components for solid composite propellants.

The presence of an unusual combination of substituents in these molecules does not allow to rely on the calculatedΔH°f in the solid state, and one has to obtain them experimentally. The energies of combustion of compounds I and II in oxygen atmosphere were measured using a static bomb calorimeter (automatic calorimeter AKS-3M).
To avoid hazardous work and ensure complete combustion, we decided to decrease the amount of compound used in each experiment and use benzoic acid as an auxiliary sample for the combustion measurements.
The standard molar enthalpies of formation, in the crystalline phase, ΔfH°m(cr) for each compound, atT= 298.15K, are given below.
For compound I, ΔfH°m(cr) = 261.5±5.0(kJ/mol)
For compound II, ΔfH°m(cr) = 246.4±6.7(kJ/mol)
Thermodynamic analysis of solid composite propellants (SCP) formulations basing on oxidizers I or II, two kinds of binder, active one AB (C18.96H34.64N19.16O29.32, ΔHf=-757kJ/kg,ρ=1.49g/cm3) and hydrocarbon one HCB (C72.15H119.2, ΔHf=-393kJ/kg,ρ=0.91g/cm3) in concentration was not less than 19%(volume fraction), and aluminum up to 18% (volume fraction) was performed. The specific impulse valuesIsp(atPc∶Pa=40∶1), and combustion temperaturesTchave been calculated using the code "High temperature thermodynamic equilibria" TERRA[16]. Values of so called effective impulsesIef(n) wheren=1, 2 or 3 accordingly the number of the rocket complex stage (1- the lower, 3- the higher) were calculated too. These values allow to compare the relative effectiveness of different SCP-formulations with different densitiesρand theIspvalues when these compositions are used in the given rocket complex stage[17]:
Ief(1)=Isp+ 100(ρ-1.9);
Ief(2)=Isp+ 50(ρ-1.8);
Ief(3)=Isp+ 25(ρ-1.7)[17];
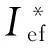
2Results and Discussions
There is a part of calculated data on formulations compound I + HCB + Al in Table 1.
The obtained data are presented more completely in Fig.1.

Table 1 Ballistic parameters of formulations compound I + HCB + Al

Fig.1 Ballistic parameters of formulations compound I + HCB + Al as function of the binder volume content and aluminum percentage

ρ=1.72 g/cm3, is already a good achievement. Such oxidizers as PA and ADN can not achieve these parameters.
Let us consider replacing HCB to the active binder AB (Table 2, Fig.2).

Table 2 Ballistic parameters of formulations compound I + AB + Al

Fig.2. Ballistic parameters of formulations compound I + AB + Al as function of the binder volume content and aluminum percentage
When replacing HCB to AB the situation has changed considerably.
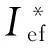
In Table 3 and Fig.3 the obtained data are represented.
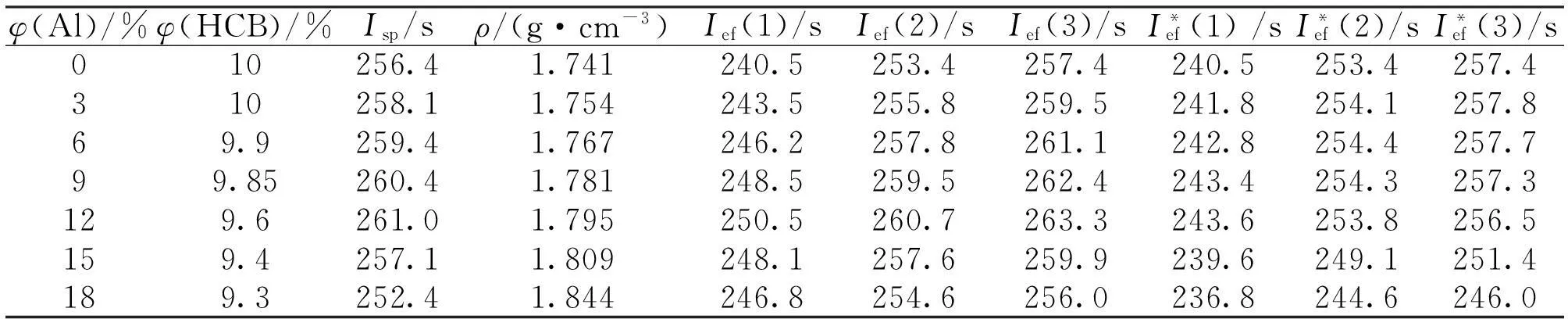
Table 3 Ballistic parameters of formulations compound II + HCB + Al at about 19% of the binder
It is evident that the formulations with compound II and HCB can provide easilyIspequal to 260 s at the Al content of 10%. ThisIspvalues is reached at the binder content about 19%(volume fraction),ρ= 1.77g/cm3(Table 3). As we saw above in the case of oxidizer I, the increase of the Al content above than 10% decreases already the ballistic parameters because the oxidizer under consideration have a relatively low α value (1.18), not so high that AP and ADN have (2.0 and higher).

Fig.3. Ballistic parameters of formulations compound II + HCB + Al as function of the binder volume content and aluminum percentage
It is important to add that if one introduces AP or ADN into formulations with I or II (a partial replacement of compound I or II with AP or ADN), it worsens the ballistic parameters. One may consider oxidizers I and II as compounds creating binary metal-free
formulations of propellants (Isp=256s atρ=1.74g/cm3) using a common HCB of 19%(volume fraction) or 10%(mass fraction). =
In case of AB (Table 4, Fig.4) in formulations with II we see the same regularities as we saw above with oxidizer I.

Table 4 Ballistic parameters of formulations compound II + AB + Al

續表4
Since the ballistic parameters of oxidizers I and II are too close we have analysed how the ballistic parameters of binary formulations (a binder + an oxidizer) depend on the nature and amount of the binder for the example of oxidizer II (Fig.5). In the case of HCB, theIef(n) values decrease considerably with the increasing of the HCB content higher than 19%(volume fraction). In case of AB, if the AB content is higher than 19% onlyIef(1) decreases slightly, whileIef(2) andIef(3) even increase until a volume binder fraction of 30%-35%.
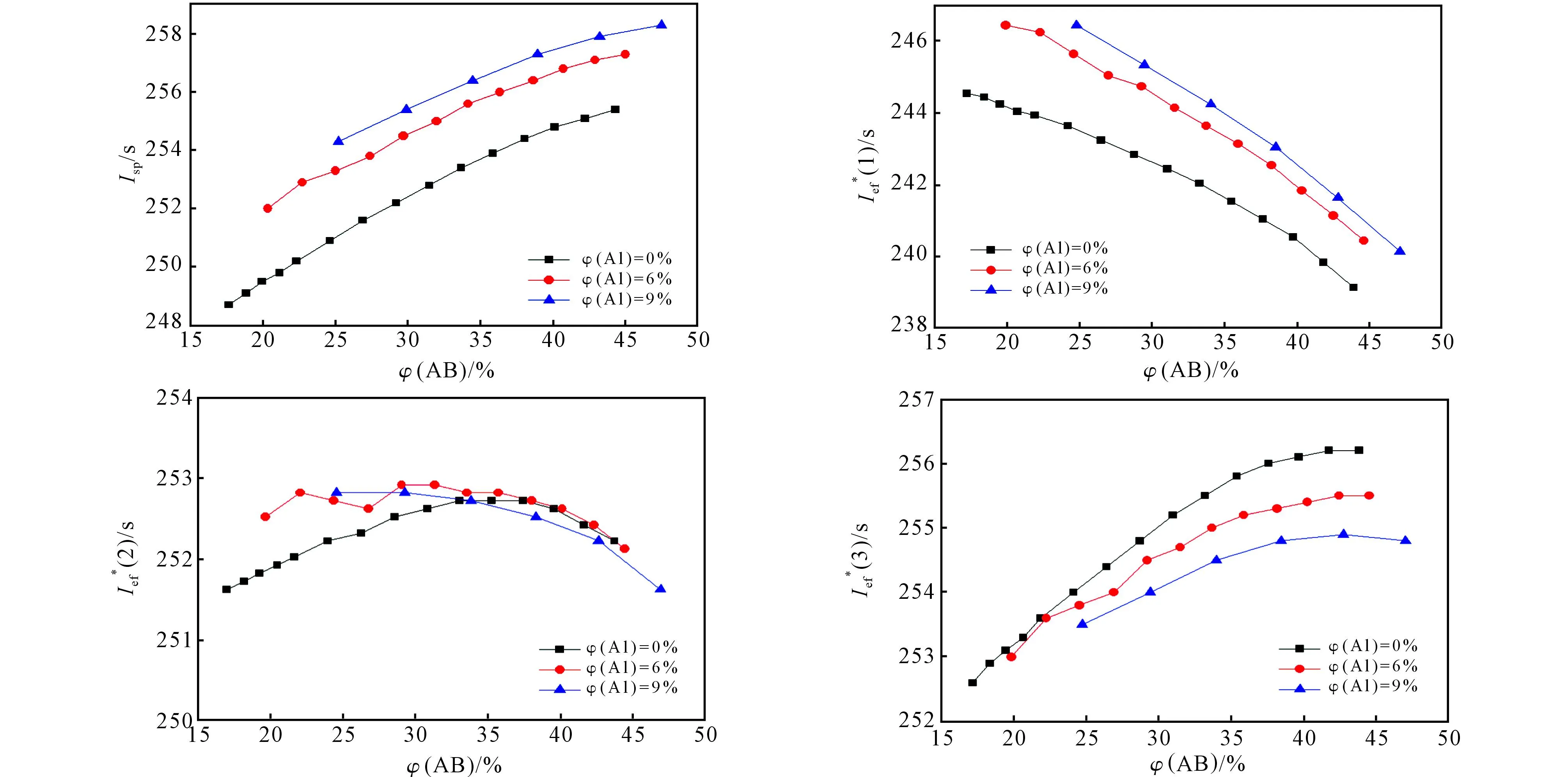
Fig.4. Ballistic parameters of formulations compound I + AB + Al as function of the binder volume content and aluminum percentage

Fig.5. Dependence of Isp and (n) of binary formulations compound II + binder upon the nature and amount of the binder
If one makes it a condition that the volume fraction of the binder not be lower than 19%, then for bottom stages the compositions with AB are better. For the second stage, the compositions with HCB are a bit better, but only at the binder percentage of 19%-19.5%. For the upper stage the compositions with HCB are considerably better, but the possibility to create the composition with oxidizer I or II with a high amount of the active binder is rather interesting because it is allowed to improve rheologic and physico-mechanical properties. In metal-free formulations oxidizers I and II are significantly better than AP or ADN (even if AP or ADN are used in formulations with Al), if we take into account the loss ofIspbecause of the presence of the solid phase in the combustion products of Al-containing compositions.
3Conclusions
(1)The experimental values of the standard enthalpy of formation of crystalline 3,4- and 3,5-dinitro-1-(trinitromethyl)-1H-pyrazoles have been obtained that are equal to (261.5±5.0)kJ/mol and (246.4±6.7)kJ/mol, correspondingly.
(2)Thermodynamic analysis showed that these compounds may be used successfully as oxidizer in solid composite propellants, especially in metal-free compositions or with a small content of metal. For the bottom stage 3,4-dinitro-1-(trinitromethyl)-1H-pyrazole is a bit better than 3,5-dinitro-1-(trinitromethyl)-1H-pyrazole, for the upper stage the both oxidizers show the equal ballistic parameters.
(3)The ability to create the metal-free solid composite propellants with the binder percentage not lower than 19%, havingIspequal to 256.5-257.0s at density equal to 1.72-1.74g/cm3may be considered as a remarkable success.
Acknowledge
The investigation was supported by the Ministry of Education and Science of the Russian Federation by agreement of 10.11.2015 № 14.613.21.0043.
References:
[1]Shevelev S A, Dalinger I L. Advances in the nitropyrazole chemistry[J]. Russian Journal of Organic Chemistry, 1998, 34(8): 1071-1080.
[2]Zaitsev A A, Dalinger I L, Shevelev S A. Dinitropyrazoles[J]. Russian Chemical Reviews, 2009, 78(7): 589-627.
[3]Dalinger I, Shevelev S, Korolev V, et al. Chemistry and thermal decomposition of trinitropyrazoles[J]. Journal of Thermal Analysis Calorimitor, 2011, 105: 509-516.
[4]Sheremetev A B, Yudin I L, Palysaeva N V, et al. The Synthesis of 4-(3-nitrofurazan-4-yl)-3,5-dinitropyrazole and its salts[J]. Journal of Heterocycl Chemistry, 2012, 49(2): 394-401.
[5]Yin P, Zhang J, Parrish D A, Shreeve J M. Energetic
N,N'-ethylene-bridged bis(nitropyrazoles): Diversified functionalities and properties[J]. Chemistry - A European Journal, 2014, 20(50): 16529-16536.
[6]Li C, Liang L, Wang K, et al. Polynitro-substituted bispyrazoles: a new family of high-performance energetic materials [J]. Journal of Material Chemistry. A 2014, (2): 18097-18105.
[7]Dalinger I L, Shakhnes A K, Monogarov K A, et al. Novel high energetic pyrazoles: N-fluorodinitromethyl and N-(difluoroamino)dinitromethyl derivatives[J]. Mendeleev Commun, 2015, 25(6): 429-431
[8]Lempert D, Nechiporenko G, Manelis G. Energetic performances of solid composite propellants[J]. Central European Journal of Energetic Materials, 2011, 8(1): 25-38.
[9]Lempert D, Nechiporeeko G, Manelis G. Influence of heat release value and gaseous combustion products content on energetic parameters of solid composite propellants .Theory and practice of energetic materials[C]// Proceedings of the 2009 International Autumn Seminar on Propellants, Explosives and Pyrotechnics. Kunming: PEP, 2009: 234-243.
[10] Smirnov Al, Lempert Da, Pivina Ta. Energetic Science & Technology in Central Europe[M]. Maryland: MD CALCE EPSC Press, 2012: 97-129.
[11] Lempert D B, Dorofeenko E M, Soglasnova S I, et al. Dependence of specific impulse and combustion temperature of solid propellants on their elemental composition and heat content[J]. Combustion, Explosion, and Shock Waves, 2012, 48(4): 424-427.
[12] Lempert D B. Preliminary estimation of the effectiveness of new and predicted energetic compounds as oxidizers for solid composite propellants [C]//17th Proceedings of the Seminar New Trends in Research of Energetic Materials. Pardubice : University of Pardubice, 2014: 281-287.
[13] Lempert D B, Dorofeenko E M. Optimum compositions of metal free energetic compositions with varying oxidizer content and nitro and difluoroamine groups in it[J]. Combustion, Explosion and Shock Waves, 2014, 50(4): 447-453.
[14] Shastin A V, Lempert D B. Energy potential of some triazine derivatives [J]. Russian Journal of Physical Chemistry B, 2014, 8(5):716-719.
[15] Dalinger L, Vatsadze I A, Shkineva T K, et al. Novel highly energetic pyrazoles: N-Trinitromethyl-substituted Nitropyrazoles[J]. Journal of Asian Chemsitry, 2015, 10(9): 1987-1996.
[16] Trusov B. The code Terra for modeling phase and Chemical Equilibria [C]// In: Proceeding of the XIV International. Symposium on Chemical Thermodynamics. St-Petersburg:[s.n],2002:483-484.
[17] Pavlovets G, Tsutsuran V. Physicochemical Properties of Powders and Propellants[M]. Moscow: Russian Ministry of Defense Publishing House, 2009: 408.
CLC number:TJ55;V512
Document Code:AArticle ID:1007-7812(2016)02-0016-06
Received date:2016-03-03;Revised date:2016-03-24
DOI:10.14077/j.issn.1007-7812.2016.02.003
Foundation:Ministry of Education and Science of the Russian Federation (14.613.21.0043)
Biography:LEMPERT David B.(1942-), male, Ph. D, Professor. Research field: Aerospace propultion. E-mail:lempert@icp.ac.ru

