力學刺激對體外立體培養軟骨細胞基質代謝的影響
段王平,苑偉,孫振偉,李琦,趙昱,衛小春
(山西醫科大學第二醫院骨科,山西 太原 030001)
實驗研究
力學刺激對體外立體培養軟骨細胞基質代謝的影響
段王平,苑偉,孫振偉,李琦,趙昱,衛小春*
(山西醫科大學第二醫院骨科,山西 太原 030001)
目的 分析周期性動態壓縮刺激對體外海藻酸鈉立體培養關節軟骨細胞基質合成代謝的影響。方法 取2月齡新西蘭白兔10只,酶解消化獲取膝關節全層軟骨細胞,以海藻酸鈉凝膠立體培養,分為實驗組和對照組兩組,對照組行靜態培養,未施加任何壓力;實驗組利用Flexcell-5000力學加載系統對體外培養軟骨細胞進行周期性壓縮應力加載,1 h/d。于加載第7、14、21天留取軟骨細胞,采用實時定量聚合酶聯反應對軟骨細胞蛋白聚糖(aggrecan,AGG)、Ⅱ型膠原、Ⅹ型膠原及基質金屬蛋白酶-13(matrix metalloproteinase-13,MMP-13)mRNA進行定量分析。結果 實驗組在第7天AGG及Ⅱ型膠原mRNA的表達明顯增高(P<0.05),隨加載時間延長,其表達量逐漸下降,在第14、21天兩組比較均無明顯差異。同時,實驗組在第7天時,Ⅹ型膠原及MMP-13的表達無明顯差異。第14天,實驗組Ⅹ型膠原及MMP-13 mRNA的表達與對照組比較明顯增高(P<0.05)。結論 立體培養軟骨細胞在生理力學刺激7 d時可明顯促進其基質合成能力,但隨刺激時間的延長,其基質合成能力逐漸減弱,軟骨細胞趨于肥大分化。
軟骨細胞;海藻酸鈉;周期性動態壓縮;代謝
目前,關節軟骨損傷患者越來越多。尤其各種創傷引起青壯年患者關節軟骨的局部損傷,治療較為困難,嚴重影響患者的生活質量。組織工程技術為促進軟骨損傷修復帶來了福音,但軟骨細胞在體外培養軟骨構建過程中極易發生失分化[1-2],從而導致軟骨損傷修復效果欠佳,修復組織力學性能不能恢復到正常軟骨的水平,且隨體內修復時間的延長,修復組織的快速退變[3-4]成為制約其發展的主要問題。大量相關研究證實,力學刺激在關節軟骨形態、細胞外基質新陳代謝及軟骨修復方面發揮著重要作用[5-6]。但力學刺激在組織工程軟骨構建中對軟骨細胞基質合成能力的影響,尚不完全明了。本課題在建立Flexcell-5000軟骨細胞力學加載系統的基礎上,通過實時定量聚合酶聯反應(real-time PCR,RT-PCR)技術分析精確控制的動態壓縮應力對體外海藻酸鈉凝膠立體培養軟骨細胞基質合成代謝能力的影響,進一步完善明確力學刺激在軟骨細胞組織工程軟骨構建中的作用。
1 資料與方法
1.1 實驗動物 2月齡新西蘭大白兔10只,均單獨籠養,正常飲水、飲食。無菌條件剖取兔雙膝關節,于超凈工作臺削取全層軟骨,絞碎,于37℃ CO2培養箱采用0.4%鏈霉蛋白酶和0.025% Ⅱ型膠原酶依次酶解消化獲取膝關節全層軟骨細胞。
1.2 海藻酸鈉凝膠立體培養 按每毫升生理鹽水加入12 mg海藻酸鈉干粉攪拌制備海藻酸鈉凝膠,0.22 μm濾網除菌。將上述細胞懸液與海藻酸鈉凝膠充分混勻吹打,保持細胞濃度為4×106/mL。制備海藻酸鈉凝膠盤,體積50 μL,圓柱狀,高度3 mm、直徑4.5 mm,10%氯化鈣溶液浸泡5 min膠化定形,移入力學加載細胞培養板,每孔加原代培養基3 mL,培養條件:37℃,5% CO2,每3天換液1次。
1.3 力學加載及分組 采用Flexcell-5000基底壓縮加載系統(美國,Flexcell公司)對海藻酸鈉凝膠盤立體培養軟骨細胞實現精確的周期性壓縮應力。本實驗分為兩組,即實驗組和對照組。對照組行靜態培養,未施加任何壓力;實驗組:對海藻酸鈉凝膠盤進行周期性壓縮應力加載,加載條件:正弦波,0.5Hz,20kPa,1 h/d。于加載第7、14、21天進行相關分析。
1.4 RT-PCR檢測 于加載第7、14、21天收集凝膠盤,,加入10%乙二胺四乙酸二鈉溶液,輕微震蕩分離獲取軟骨細胞。Trizol法提取總RNA,使用PrimeScriptTM RT試劑盒將RNA反轉錄為cDNA,采用PCR儀將cDNA進行相應引物擴增。引物設計如下:蛋白聚糖(aggrecan,AGG):5′-TCTACCGCTGTGAGGTGATGC-3′和5′-TTCACCACGACCTCCAAGG-3′;Ⅱ型膠原:5′-ACACTGCCAACGTCCAGATG-3′和5′-GTGATGTTCTGGGAGCCCTC-3′;Ⅹ型膠原:5′-AGCCAGGGTTGCCAGGACC-3′和5′-CCAGGAGCACCATATCCTGT-3′;基質金屬蛋白酶-13(matrix metalloproteinase-13,MMP-13):5′-CACCGGATCTGCCAAGAGA-3′和5′-CTGGAGAACGTGATTGGAGTCA-3′;GAPDH:5′-GAGCCCTTCCACAATGCCAAA-3′和5′-GTCGTGGAGTCTACTGGTGTC-3′。通過計算機軟件測量和計算Ct值,計算目的基因相對轉錄水平[7]。
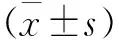
2 結 果
與對照組比較,實驗組在第7天AGG及Ⅱ型膠原mRNA的表達明顯增高(P<0.05),隨加載時間延長,其表達量逐漸下降,在第14、21天兩組比較均無明顯差異(見圖1)。與對照組比較,實驗組在早期第7天時,Ⅹ型膠原及MMP-13的表達無明顯差異。在第14天時,實驗組Ⅹ型膠原及MMP-13 mRNA的表達與對照組比較明顯增高(P<0.05)。而在第21天時兩組之間Ⅹ型膠原及MMP-13 mRNA的表達無明顯差異(見圖2)

a AGG b Ⅱ型膠原
圖1 AGG及Ⅱ型膠原mRNA表達柱狀圖
3 討 論
軟骨細胞作為成體組織來源的組織工程軟骨種子細胞,由于軟骨組織來源缺乏,體外大量的擴增培養是目前常用的方法之一[8]。但軟骨細胞在體外單層擴增培養的方式極易喪失其分化成熟的表型,基質合成能力降低,退變轉化為纖維細胞表型[9]。本課題組前期通過微管吸吮力學分析進一步證實,老年和骨關節炎軟骨細胞黏彈性力學特性明顯降低[10]。且在體外培養擴增過程中,隨著傳代次數增加,軟骨細胞逐漸失去黏彈性蠕變特性,表現為彈性固體特征,且隨傳代次數增加,軟骨細胞楊氏模量和表面張力逐漸增高,細胞質內細胞骨架(cytoskeleton,CSK)的空間分布、成分及含量發生明顯變化,提示CSK在軟骨細胞體外培養失分化過程中的作用[11-12]。
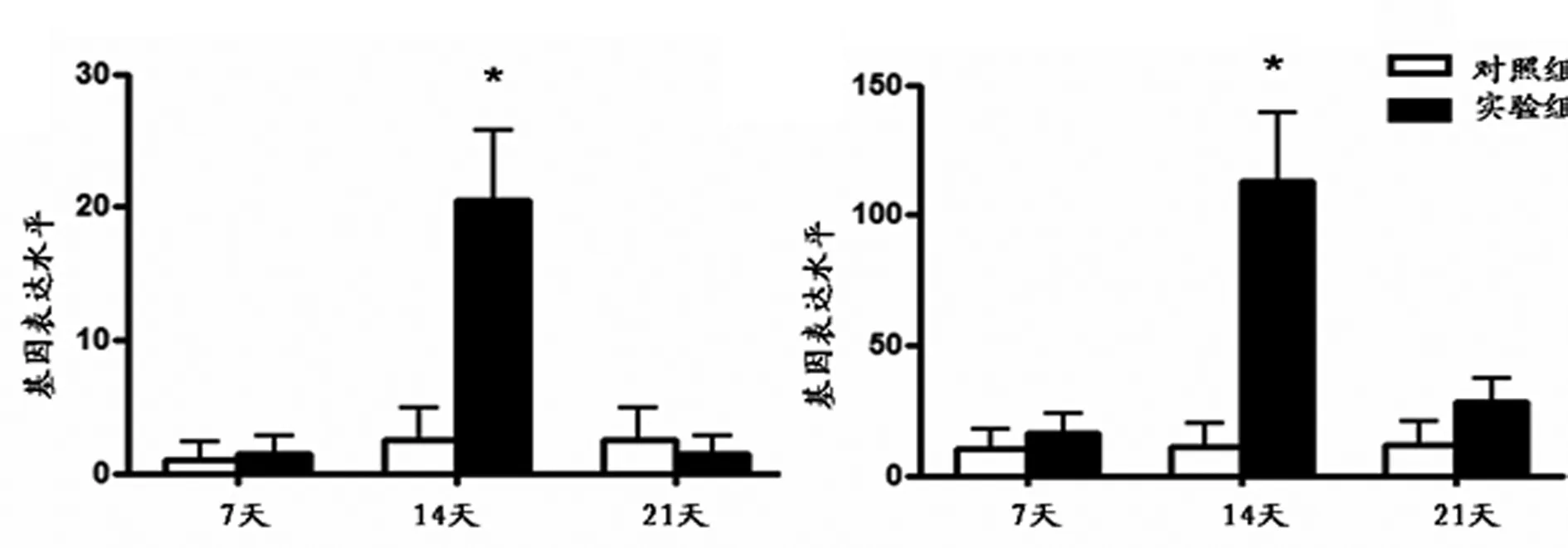
a X型膠原 b MMP-13
圖2 X型膠原及MMP-13 mRNA表達柱狀圖
大量研究證實,藻酸鈉微球等體外三維立體培養可維持軟骨細胞的表型,增強軟骨細胞特異性Ⅱ型膠原和糖胺聚糖類的表達[13]。同時,有研究發現,體外立體培養可明顯促進骨髓間充質干細胞(bone mesenchymal stem cells,BMSCs)向軟骨細胞的分化[14]。且立體三維培養能使已經去分化的軟骨細胞發生重分化,穩定其表型,恢復去分化軟骨細胞表達特異性標志物的表達,恢復其超微結構和維持Ⅰ、Ⅱ膠原表達的作用[15-16]。
在日常活動中,人體關節軟骨承受著壓力、剪切力等多種力學刺激。大量研究證實,力學環境對軟骨細胞生長、分化、表型的維持,對組織工程軟骨的修復及重建有非常重要的作用[17-18]。本實驗對體外海藻酸鈉凝膠立體培養軟骨細胞施加0.5Hz、20kPa的周期性動態壓縮應力,根據力學計算,這種壓力更加接近于關節軟骨正常狀態下的生理壓力。通過實驗進一步證實,壓縮刺激7天后軟骨細胞AGG和Ⅱ型膠原基因表達水平明顯上調,這說明了適當力學刺激促進提高了軟骨細胞基質合成能力。但隨著力學刺激時間的延長,軟骨細胞趨于肥大方向分化,主要表現為X型膠原及MMP-13基因表達的提高。有研究表明,軟骨細胞可通過細胞骨架肌動蛋白的解聚,使細胞本身力學和機械敏感性適應周圍的力學環境的變化[19]。長時間超負荷的力學加載,導致細胞骨架的改變,致使軟骨細胞肥大適應力學刺激基質合成能力的退變[20]。
本實驗通過精確的周期性壓縮應力加載定量分析發現,海藻酸鈉立體培養軟骨細胞在生理壓力刺激7天d時可明顯促進其基質合成能力。但隨力學刺激時間的延長,其基質合成能力逐漸減弱,軟骨細胞趨于肥大分化,完善了力學刺激在軟骨細胞組織工程軟骨構建中的作用。
[1]Danisovic L,Varga I,Zamborsky R,etal.The tissue engineering of articular cartilage:cells,scaffolds and stimulating factors[J].Exp Biol Med (Maywood),2012,237(1):10-17.
[2]Goepfert C,Slobodianski A,Sch illing AF,etal.Cartilage engineering from mesenchymal stem cells[J].Adv Biochem Engin/Biotechnol,2010(123):163-200.
[3]Henson FM,Getgood AM,Caborn DM,etal.Effect of a solution of hyaluronic acid-chondroitin sulfate-N- acetyl glucosamine on the repair response of cartilage to single-impact load damage[J].Am J Vet Res,2012,73(2):306-312.
[4]Buckley CT,Meyer EG,Kelly DJ.The influence of construct scale on the composition and functional properties of cartilaginous tissues engineered using bone marrow-derived mesenchymal stem cells[J].Tissue Eng Part A,2012,18(3-4):382-396.
[5]Croos JA,Dhaliwal SS,Grynpas MD,etal.Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic.gene changes in chomdrocytes resulting in increased extracellular matrix accumulation[J].Matrix Biology,2006,25(6):323-331.
[6]Trickey WR,Vail TP,Guilak F.The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes[J].J Orthop Res,2004,22(1):131-139.
[7]Wei L,Kanbe K,Lee M,etal.Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation[J].Developmental Biology,2010,341(1):236-245.
[8]Binette F,McQuaid DP,Haudenschild DR,etal.Expression of a stable articular cartilage phenotype without evidence of hypertrophy by adult human articular chondrocytes in vitro[J].J Orthop Res,1998,16(2):207-216.
[9]Bonaventure J,Kadhom N,Cohen-solal L,etal.Reexpression of cartilage-specific genes by dedifferentialted human articular chondrocytes cultures in alginate beads[J].Exp Cell Res,1993,212(1):97-104.
[10]Duan WP,Sun ZW,Li Q,etal.Normal age-related viscoelastic properties of chondrons and chondrocytes isolated from rabbit knee[J].Chin Med J,2012,125(14):2574-2581.
[11]Duan W,Wei L,Zhang J,etal.Alteration of viscoelastic properties is associated with a change in cytoskeleton components of ageingchondrocytes from rabbit knee articular cartilage[J].Mol Cell Biomech,2011,8(4):253-274.
[12]段王平.不同年齡兔膝關節軟骨單位生物學特性分析[D].太原:山西醫科大學博士論文,2011.
[13]Takayama K,Ishida K,Matsushita T,etal.SIRT1 regulation of apoptosis of human chondrocytes[J].Arthritis Rheum,2009,60(9):2731-2740.
[14]Cheng NC,Estes BT,Awad HA,etal.Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix[J].Tissue Eng Part A,2009,15(2):231-241.
[15]Schuurman W,Gawlitta D,Klein TJ,etal.Zonal chondrocyte subpopulations reacquire zone-specific characteristics during in vitro rediffer-entiation[J].Am J Sports Med,2009,37(1):97-104.
[16]Carossino AM,Recenti R,Carossino R,etal.Methodological models for in vitro amplification and maintenance of human articular chondrocytes from elderly patients[J].Biogerontology,2007,8(5):483-498.
[17]Hoenig E,Winkler T,Gabriela M,etal.High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue engineered cartilage[J].Tissue Eng Part A,2011,17(9-10):1401-1411.
[18]Huang AH,Farrell MJ,Kim M,etal.Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stemcell-laden hydrogel[J].Eur Cell Mater,2010,26(19):72-85.
[19]Guilak F,Erickson GR,Ting-Beall PH.The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes[J].Biophys J,2002,82(2):720-727.
[20]Campbell JJ,Blain EJ,Chowdhury TT,etal.Loading alters actin dynamics and up-regulates cofilin gene expression in chondrocytes[J].Biochem Biophys Res Commun.2007,361(2):329-334.
Metabolize Analysis of Chondrocytes Cultured in Alginate in Response to Cyclical Dynamic Compression
Duan Wangping,Yuan Wei,Sun Zhenwei,etal
(Department of Orthopaedics,the Second Hospital of Shanxi Medical University,Taiyuan 030001,China)
Objective To analyze the matrix metabolite of isolated chondrocytes cultured in alginate in response to cyclical dynamic compression.Methods The chondrocytes were isolated from the 2-months rabbit knees cartilage,and the chondrocytes were cultured in alginate.The cells were treated with cyclical dynamic compression by using Flexcell-5000 compression system kept under an uncompressed condition as a control.The mRNA gene expression of aggrecan (AGG),type Ⅱcollagen,type X collagen and matrix metalloproteinases 13 (MMP-13) were determined by reverse transcription polymerase chain reaction (RT-PCR) at 7,14 and 21 day after under loading.Results A significant up-regulation was observed in the gene expression of AGG and type Ⅱcollagen on 7 day in the experimental group compared to control group,but showed no significant difference on 14 and 21 days.The type X collagen and MMP-13 mRNA gene expression were significantly increased on 14 days in the experimental group compared to control group.There were no significant difference on 7 and 21 days.Conclusion Chondrocytes cultured in alginate can keep their matrix synthetic ability under the physiological compression early,but the cells degenerate and hypertrophy over loading time.
chondrocytes;alginate;cyclical dynamic compression;metabolize
國家自然科學基金項目(31340010、31271033);教育部高等學校博士學科點專項科研基金聯合資助課題(20121417120004);山西省基礎研究計劃項目青年科技研究基金項目(2013021036-3);高等學校科技創新項目(20131105);山西醫科大學博士啟動基金項目(03201114);*本文通訊作者:衛小春
1008-5572(2015)05-0428-04
R318.01
A
2014-04-10
段王平(1981- ),男,講師,山西醫科大學第二醫院骨科,030001。

