90例胃腸間質瘤的外科診治分析
薛勇敢,張秉棟,李 鵬,劉洪一,賈寶慶
1解放軍醫學院,北京 100853;2解放軍總醫院 腫瘤外科,北京 100853
臨床研究論著
90例胃腸間質瘤的外科診治分析
薛勇敢1,張秉棟1,李 鵬2,劉洪一2,賈寶慶2
1解放軍醫學院,北京 100853;2解放軍總醫院 腫瘤外科,北京 100853
目的探討胃腸間質瘤(gastrointestinal stromal tumor,GIST)的臨床特點、診療方法和預后,進一步提升對此類疾病的認識。方法回顧分析解放軍總醫院腫瘤外科2009年9月-2014年9月收治的90例GIST患者的完整臨床和病理資料。結果最常見的臨床表現為腹部脹痛不適、納差占41.1%(37/90),其次為消化道出血占20.0%(18/90),腹部包塊占4.4%(4/90),腸梗阻癥狀占3.3%(3/90),28例無明顯癥狀占31.1%,為檢查時偶然發現。術前診斷主要依靠胃腸鏡、超聲內鏡、CT、MRI等影像學檢查。90例均接受手術治療。免疫組化CD117陽性率90.0%(81/90),CD34陽性率91.1%(82/90),S-100陽性率11.1%(10/90),SMA陽性率33.3%(30/90)。中位隨訪時間27.4(1 ~ 60)個月,8例復發或轉移,3例再次手術,2例因腫瘤進展死亡。單因素分析顯示,腫瘤大小(P=0.019)及核分裂數目(P=0.002)是影響預后的因素。結論GIST的臨床表現無特異性,胃腸鏡、超聲內鏡、CT、MRI等檢查有助于該病術前診斷。確診主要依靠病理學及免疫組化,外科手術是GIST的首選治療方式。
胃腸道間質瘤;外科手術;病理學
胃腸間質瘤(gastrointestinal stromal tumors,GIST)是消化道最常見的間葉組織腫瘤,起源于胃腸道Cajal間質細胞[1-2]。GIST易被誤診為平滑肌或神經源性腫瘤。但GIST與平滑肌或神經源性腫瘤在發病機制、生物學行為、病理特征、治療方法及預后方面均有很大不同[3-4]。本研究通過回顧分析90例GIST患者的臨床和病理資料,探討GIST臨床特點、診療方法和預后,旨在為進一步提升此類疾病的診治水平、改善預后提供幫助。
資料和方法
1 臨床資料 收集本院腫瘤外科2009年9月-2014年9月收治且病理確診為GIST的有完整臨床和病理資料患者90例,男48例(53.3%),女42例(46.7%);年齡21 ~ 84歲,平均年齡59.1歲。間質瘤位于胃69例(76.7%),小腸16例(17.8%),結直腸5例(5.6%)。
2 方法 回顧性分析90例GIST患者的病歷資料,探討GIST臨床表現、影像學檢查、病理、免疫組化結果及手術治療方式。
3 隨訪 本組90例采用門診隨診及電話隨訪,末次隨訪時間為2014年10月31日。主要觀察患者的復發、轉移和死亡情況。共有86例獲得隨訪,失訪4例,隨訪率95.6%。
4 統計學方法 應用SPSS19.0統計軟件對本組資料進行分析。對可能影響預后的因素采用Kaplan-Meier生存分析和Log-rank時序檢驗。P<0.05為差異有統計學意義。
結 果
1 臨床表現 腹部脹痛不適、納差37例(41.1%),消化道出血18例(20.0%),腹部包塊4例(4.4%),腸梗阻癥狀3例(3.3%),無癥狀查體或合并其他疾病檢查時偶然發現28例(31.1%)。
2 術前影像學檢查 54例胃腸鏡檢查考慮為GIST,其中18例超聲內鏡檢查考慮為GIST。25例CT檢查考慮為GIST。6例MRI檢查考慮為GIST。4例腹部彩超考慮為GIST。1例上消化道造影考慮為GIST。8例胃腸鏡檢查取病理考慮為GIST,術前依靠影像學診斷符合共38例(42.2%),其余患者診斷為胃息肉、腹腔轉移瘤、平滑肌瘤及神經源性腫瘤等。
3 手術治療 本組90例均行手術切除治療,切緣均為陰性,無手術死亡及術后嚴重并發癥。69例胃間質瘤中40例行腹腔鏡下胃楔形切除,其中腹腔鏡胃鏡雙鏡聯合切除3例,聯合膽囊切除2例;6例行腹腔鏡輔助近端胃大部切除術,其中聯合膽囊切除1例;1例行腹腔鏡輔助遠端胃大部切除術;2例行腹腔鏡輔助全胃切除術;20例行開腹胃腫瘤切除術。16例小腸間質瘤中,14例行開腹小腸腫瘤切除術;2例行腹腔鏡下小腸腫瘤切除術,其中聯合膽囊切除1例。5例結直腸間質瘤中,1例行乙狀結腸腫瘤切除術,1例經腹會陰聯合直腸切除術,1例經腹直腸前切除術,2例經肛門腫物切除術。
4 病理診斷 術后標本行病理檢查和免疫組化均證實為GIST。大體形態:腫塊呈圓形、類圓形、結節狀或分葉狀,質韌,切面灰白或灰紅色,中心可伴有出血、壞死、囊性變。良性者多有完整包膜,邊界清楚,惡性者可侵及黏膜、肌層或周圍組織,邊界欠清。腫瘤組織由梭形細胞、上皮樣細胞構成或二者兼有,呈長條束狀排列、交織樣排列或旋渦狀排列。梭形細胞形態較一致, 呈長梭形,細胞質豐富,嗜酸性,細胞核呈桿狀或長梭形。上皮樣細胞體積較大,呈卵圓形或多角形,細胞質空亮或微嗜酸性,細胞核的多形性與核分裂數呈正比(圖1,圖2)。本組病例腫瘤最大徑0.6 ~ 16.0 cm,平均直徑6.2 cm,其中直徑≤2 cm 6例,2.1 ~ 5.0 cm 39例,5.1 ~ 10.0 cm 31例,>10 cm 14例。核分裂計數≤5個/50 HPF 66例,6 ~ 10個/50 HPF 8例,>10個/50 HPF 16例。免疫組織化學特征:CD117陽性者81例(90.0%),CD34陽性者82例(91.1%),S-100陽性者10例(11.1%),SMA陽性表達30例(33.3%)(圖3,圖4,圖5,圖6)。CD117、CD34、S-100、SMA表達情況與腫瘤部位關系見表1。

表1 不同部位 GIST 與免疫組化的關系Tab. 1 Relationship between tumor site and immunohistochemical results (n, %)
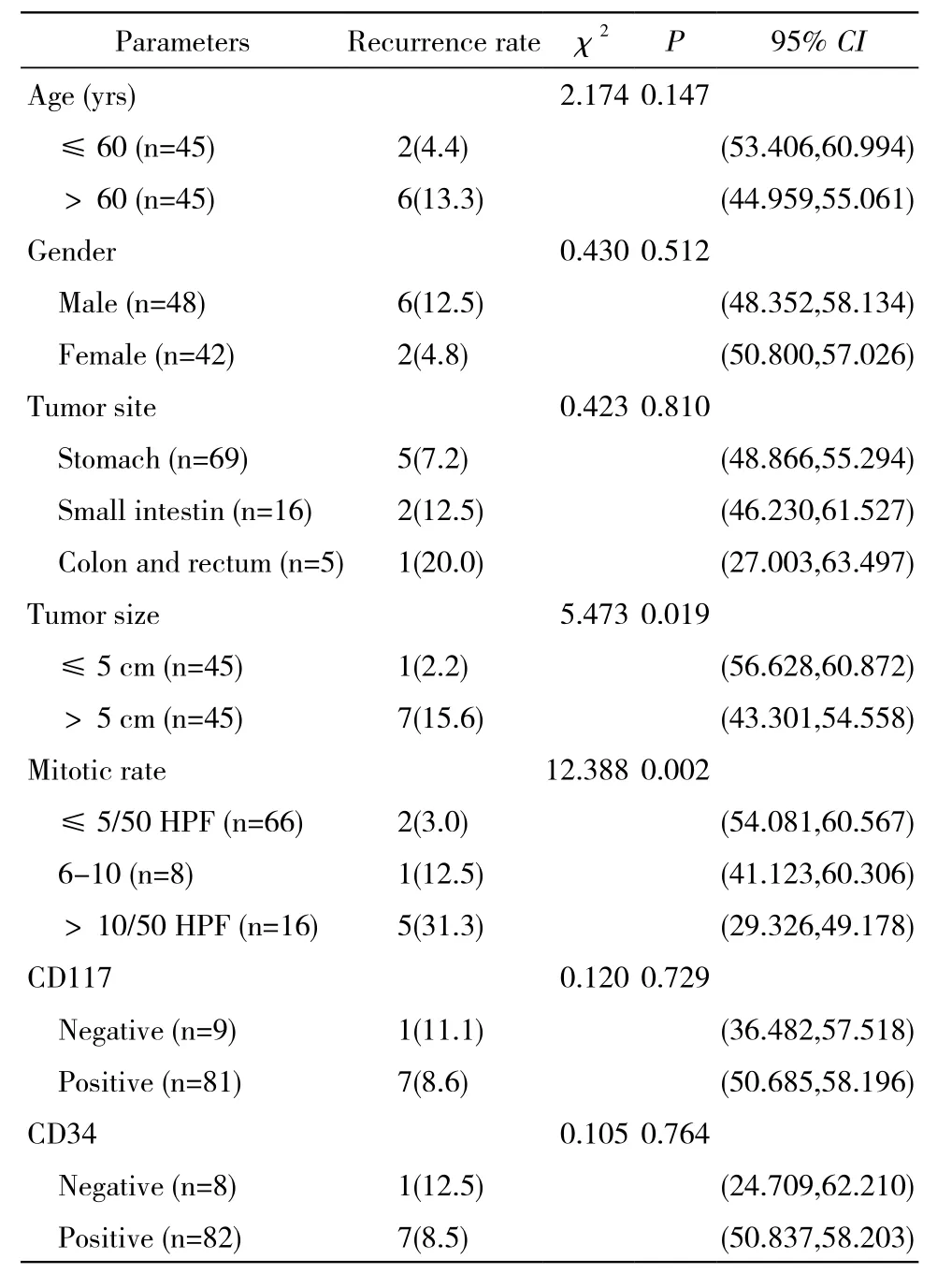
表2 影響預后的單因素分析Tab. 2 Univariate analysis of prognosis (n,%)
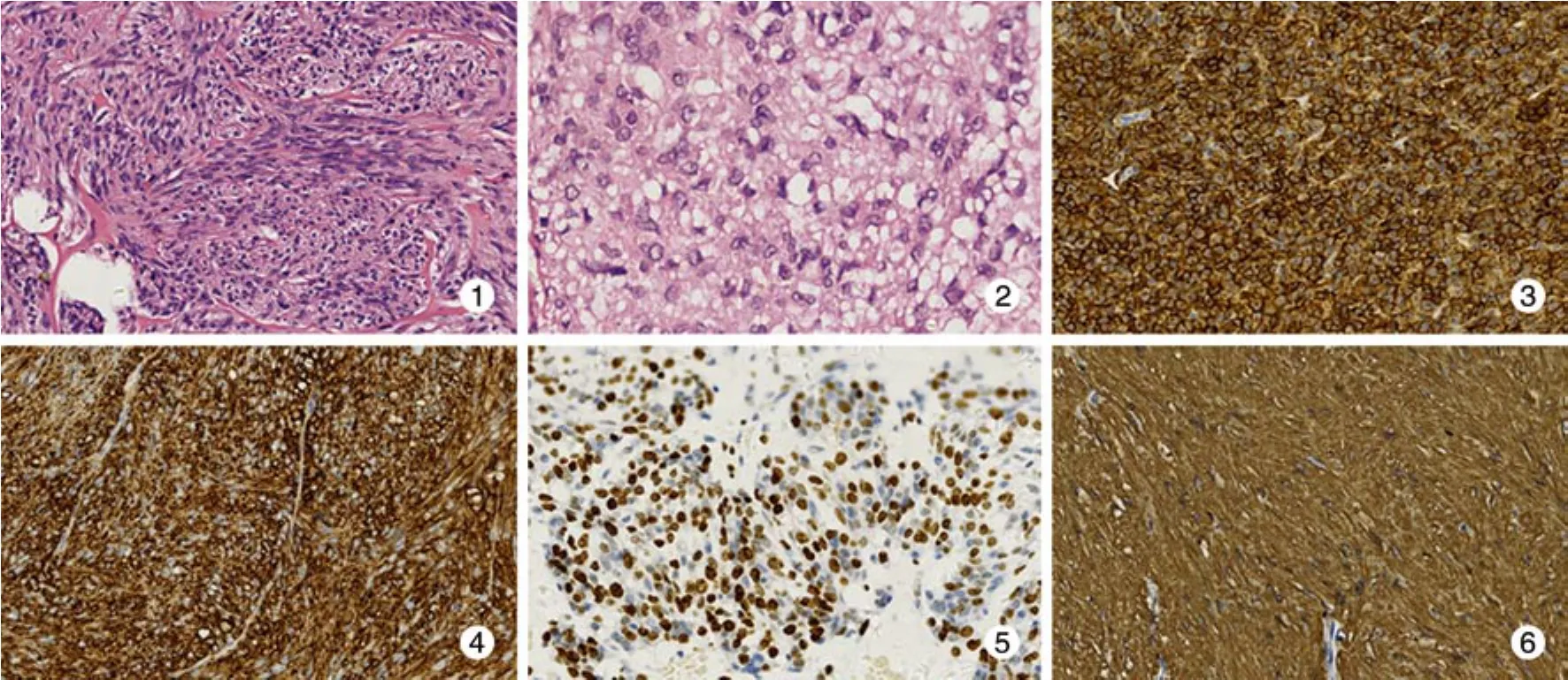
圖 1 腫瘤組織由梭形細胞和上皮樣細胞構成,呈交織樣或旋渦狀排列(HE, ×200)圖 2 腫瘤細胞核分裂象數目較多 (HE,×400)圖 3 免疫組化顯示 CD117 染色陽性 (Envision×200)圖 4 免疫組化顯示 CD34 染色陽性 (Envision×200)圖 5 免疫組化顯示 S-100 染色灶狀陽性 (Envision×200)圖 6 免疫組化顯示 SMA 染色陽性 (Envision×200)Fig. 1 Tumor composing of spindle cells and epithelioid cells (HE,×200)Fig. 2 Tumor cells exhibit high mitotic rate (HE,×400)Fig. 3 Immunohistochemical results showing the positive expression of CD117 (Envision×200)Fig. 4 Immunohistochemical results showing the positive expression of CD34 (Envision×200)Fig. 5 Immunohistochemical results showing the positive expression of S-100 (Envision×200)Fig. 6 Immunohistochemical results showing the positive expression of SMA (Envision×200)
5 隨訪 本組隨訪1 ~ 60個月,平均隨訪27.4個月,隨訪期內單純局部復發3例,局部復發合并腹腔轉移2例,單純肝轉移2例,肝、肺同時轉移1例。再次手術3例,因腫瘤進展死亡2例。
6 單因素分析顯示 腫瘤直徑>5 cm,核分裂象數目>5個/50 HPF的患者復發危險性較高,差異有統計學意義(P<0.05)。年齡、性別、腫瘤部位、CD34、CD117對預后影響不大,差異無統計學意義(P>0.05)。見表2。
討 論
GIST在世界范圍內的每年發病率為1 ~ 20/100 000,主要發生于50歲以上的中老年人,男性與女性患病比例無明顯差異[5-6]。本組病例中男性48例,女性42例,男性稍多于女性;年齡21 ~84歲,中位年齡59.1歲,與文獻報道大致相同。GIST可發生在消化道的任何部位,以胃部最常見,占60% ~ 70%,其次為小腸,占20% ~ 30%,結腸和直腸約占5%,食管<5%[7]。GIST還可發生于消化道以外部位如腸系膜和大網膜等[8]。本組資料仍以胃為高發部位76.7%(69/90),其次是小腸17.8%(16/90),最少見為結直腸5.6%(5/90)。
GIST的臨床癥狀無特異性,主要與腫瘤的部位、大小有關。患者多表現為腹部脹痛不適、消化不良等非特異癥狀,其次以消化道出血、貧血為首發癥狀。腫瘤較大者可觸及的腹部腫塊。部分患者因出現腸梗阻而急癥就診。約20%患者查體時發現。20% ~ 30%患者首診時已發生轉移,最常轉移至肝和腹膜,而淋巴結及腹部以外臟器轉移少見。本組符合以上特征。
GIST的初步診斷主要依靠腹部彩超、胃腸鏡、EUS、CT、MRI等。GIST在彩超下常顯示低回聲團塊,難以與其他疾病鑒別,定性困難。胃腸鏡可見伴有光滑、完整及正常黏膜覆蓋的隆起性病灶,頂端可有潰瘍;超聲內鏡能顯示腫瘤形態、大小及起源層次;由于腫瘤多位于黏膜下且通常不破壞消化道黏膜,活檢檢出率低。本組患者行胃腸鏡檢出54例(60.0%),其中超聲內鏡檢出18例(20.0%),8例經胃腸鏡檢查取病理考慮為GIST。CT、MRI能顯示腫瘤的大小、部位、生長方式、腫瘤與周圍臟器的關系、是否發生淋巴結和遠處轉移等情況,本組患者行CT檢出25例(27.8%),MRI檢出6例(6.7%)。
GIST的確診和分型主要依靠術后的病理組織學檢查和免疫組化分析[9]。CD117是一種酪氨酸激酶跨膜受體蛋白,是目前被公認為最具特征性的GIST免疫表型標記,在GIST中呈現出特異性彌漫性高表達,陽性表達率為80% ~ 100%,而在平滑肌腫瘤及神經鞘瘤中不表達,對GIST的診斷具有良好的敏感性和特異性。CD34是一種骨髓前體細胞標記物,單獨使用時其敏感性不高,常與CD117進行聯合鑒別,而CD34在平滑肌瘤和神經鞘瘤中也不表達。S-100為神經源性免疫標記,在GIST表達率很低,而SMA為肌源性免疫標記,在胃腸間質瘤中可有部分陽性表達。本組研究結果表明,在90例GIST患者中,CD117陽性率為90.0%,CD34陽性率為91.1%,S-100陽性率為11.1%,SMA陽性率為33.3%。
GIST對放療、化療均不敏感,手術完整切除是唯一有治愈可能的治療手段。GIST很少浸潤周圍組織器官及發生淋巴結轉移,無須常規行擴大切除和系統淋巴結清掃[10-11]。隨著微創技術的發展,腹腔鏡外科逐漸應用于GIST的治療[12-14]。對于體積較小或特殊部位的GIST,腹腔鏡與胃鏡雙鏡聯合能發揮獨特的優勢[15-18]。術中關鍵是保證腫瘤完全切除,避免瘤體破裂、腹膜種植和血行轉移,這也是影響GIST預后的重要因素[19]。本組90例均行手術完整切除腫瘤,其中69例胃間質瘤中,49例行腹腔鏡手術,20例行開腹手術;16例小腸間質瘤中,2例行腹腔鏡手術,14例行開腹手術;5例結直腸間質瘤均在直視下手術。無手術死亡及術后嚴重并發癥。本組研究表明,外科手術仍是治療GIST首選方法,腹腔鏡外科逐漸成為GIST主要治療手段,尤其是在胃間質瘤的治療方面。
腫瘤大小和核分裂象是預測GIST預后的獨立危險因素[20-21]。腫瘤越大,預后越差;核分裂象計數越多,提示腫瘤細胞的增殖活性越大,惡性程度也越高,預后也越差。本組8例出現復發或轉移患者中,腫瘤直徑>10 cm同時核分裂象>5/50 HPF的患者共6例。說明臨床上腫瘤大小和核分裂象預測GIST的預后合理、可行。
1 Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology[J]. Nat Rev Cancer,2011, 11(12): 865-878.
2 Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach[J]. Hum Pathol, 2002, 33(5): 459-465.
3 Appelman HD. Mesenchymal tumors of the gut: historical perspectives, new approaches, new results, and does it make any difference?[J]. Monogr Pathol, 1990, (31):220-246.
4 Kitamura Y. Gastrointestinal stromal tumors: past, present, and future[J]. J Gastroenterol, 2008, 43(7): 499-508.
5 Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era - A population-based study in western Sweden[J]. Cancer, 2005, 103(4): 821-829.
6 Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: Results of a nation-wide study[J]. Eur J Cancer, 2005, 41(18): 2868-2872.
7 Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition,clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis[J]. Virchows Arch, 2001, 438(1): 1-12.
8 Llenas-Garcia J, Guerra-Vales JM, Moreno A, et al. Primary extragastrointestinal stromal tumors in the omentum and mesentery:A clinicopathological and immunohistochemical study[J]. Hepatogastroenterology, 2008, 55(84): 1002-1005.
9 Dei Tos AP, Laurino L, Bearzi I, et al. Gastrointestinal stromal tumors: the histology report[J]. Dig Liver Dis, 2011, 43(S4):S304-S309.
10 Gold JS, G?nen M, Gutiérrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis[J]. Lancet Oncol, 2009, 10(11):1045-1052.
11 Woodall IC, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system[J]. Arch Surg, 2009, 144(7): 670-678.
12 Matlok M, Stanek M, Pedziwiatr M, et al. Laparoscopic Surgery In The Treatment of Gastrointestinal Stromal Tumors[J]. http://sjs. sagepub.com/cgi/pmidlookup?view=long&pmid=25452425.
13 Lin J, Huang C, Zheng C, et al. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): a size-matched comparison[J]. Surg Endosc,2014, 28(9):2577-2583.
14 Masoni L, Gentili I, Maglio R, et al. Laparoscopic resection of large gastric GISTs: feasibility and long-term results[J]. Surg Endosc,2014, 28(10):2905-2910.
15 Hiki N, Nunobe S, Matsuda T, et al. Laparoscopic endoscopic cooperative surgery[J]. Dig Endosc, 2015, 27(2):197-204.
16 Kang WM, Yu JC, Ma ZQ, et al. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors[J]. World J Gastroenterol, 2013, 19(34):5720-5726.
17 Dong HY, Wang YL, Li J, et al. New-style laparoscopic and endoscopic cooperative surgery for gastric stromal tumors[J]. World J Gastroenterol, 2013, 19(16):2550-2554.
18 Shen C, Chen H, Yin Y, et al. Endoscopic versus open resection for small gastric gastrointestinal stromal tumors: safety and outcomes[J]. Medicine (Baltimore), 2015, 94(1):e376.
19 Chen K, Zhou YC, Mou YP, et al. Systematic review and metaanalysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach[J]. Surg Endosc,2015, 29(2):355-367.
20 Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST)[J]. Cancer, 2008, 112(3):608-615.
21 Zhao WY, Xu J, Wang M, et al. Evaluation of high-risk clinicopathological indicators in gastrointestinal stromal tumors for prognosis and imatinib treatment outcome[J]. BMC Gastroenterol,2014, 14: 105.
Surgical diagnosis and treatment of gastrointestinal stromal tumors: An Analysis of 90 cases
XUE Yonggan1, ZHANG Bingdong1, LI Peng2, LIU Hongyi2, JIA Baoqing2
1Chinese PLA Medical School, Beijing 100853, China;2Department of Surgical Oncology, Chinese PLA General Hospital, Beijing 100853, China
JIA Baoqing. Email: baoqingjia@126.com
ObjectiveTo identify clinical characteristics, diagnosis, treatment and prognosis of gastrointestinal stromal tumor (GIST) and improve the level on standardized diagnosis and treatment of this disease.MethodsClinical and pathological data about 90 patients with GIST in the department of surgical oncology, Chinese PLA General Hospital from September 2009 to September 2014 were retrospectively analyzed.ResultsThe most common primary symptom was abdominal discomfort accounting for 41.1% (37/90), followed by gastrointestinal tract hemorrhage for 20.0% (18/90), abdominal mass for 4.4% (4/90), intestinal obstruction for 3.3% (3/90), 28 cases with no symptoms accounting for 31.1%. The preoperative imageological examinations comprised endoscope, endoscopic ultrasonography, CT, MRI. 90 patients received complete surgical resection. Immunohistochemistry demonstrated that tumor cells were positive for CD117 in 81 cases (90.0%) , for CD34 in 82 cases (91.1%), for S-100 in 10 cases (11.1%) and for SMA in 30 cases (33.3%). During a mean follow-up period of 27.4 (range, 1-60) months, 8 patients experienced relapse and metastasis, 3 patients underwent re-operation. Two patients died of tumor progression. Univariate analysis revealed that tumor size (P=0.019) and mitotic rate (P=0.002) were factors affecting prognosis.ConclusionGIST shows typical clinical features. Endoscopy, ultrasound endoscope, CT and MRI examination are helpful for preoperative diagnosis of this disease. The confirmation of GIST also require histopathologic examination and immunohistochemistry. Surgery treatment is the first choice for GIST.
gastrointestinal stromal tumor; surgical procedure; pathology
R 730.4
A
2095-5227(2015)06-0525-04
10.3969/j.issn.2095-5227.2015.06.001
時間:2015-03-16 11:12
http://www.cnki.net/kcms/detail/11.3275.R.20150316.1112.001.html
2015-01-04
國家“863”計劃項目(2013YQ030651)
Supported by “863” Program of China(2013YQ030651)
薛勇敢,男,在讀碩士。研究方向:胃腸腫瘤的外科治療。Email: 892407021@qq.com
賈寶慶,男,主任醫師,碩士生導師。Email: baoqingjia @126.com

