H2SO4/AC無溶劑催化合成香豆素衍生物*
肖東彩
(銀川能源學院,寧夏銀川 750105)
H2SO4/AC無溶劑催化合成香豆素衍生物*
肖東彩
(銀川能源學院,寧夏銀川 750105)
以濃硫酸改性活性炭[H2SO4/AC(Cat)]為催化劑,在無溶劑條件下,取代酚和乙酰乙酸甲酯(2)經Pechmann縮合反應合成了5個香豆素衍生物,其結構經1H NMR,13C NMR和IR確證。以間甲酚(1a)和2合成4,7-二甲基香豆素(3a)為例,考察原料配比[r=n(1a)∶n(2)]、反應溫度、Cat用量及反應時間對Pechmann反應的影響。在最佳反應條件[1a 5 mmol,r=1.0∶2.0,Cat 18%,于120℃反應3 h]下,3a收率80%。
H2SO4/AC;Pechmann反應;無溶劑反應;催化劑;合成

為了克服以上方法的不足,同時為了深入并完善本課題組對煤基活性炭(AC)催化材料的開發研究工作,本文以濃硫酸改性活性炭[H2SO4/ AC(Cat)]為催化劑,在無溶劑條件下,取代酚(1a~1e)和乙酰乙酸甲酯(2)經Pechmann縮合反應合成了5個香豆素衍生物(3a~3e,Scheme 1),其結構經1H NMR,13C NMR和IR確證。并對合成工藝進行優化。
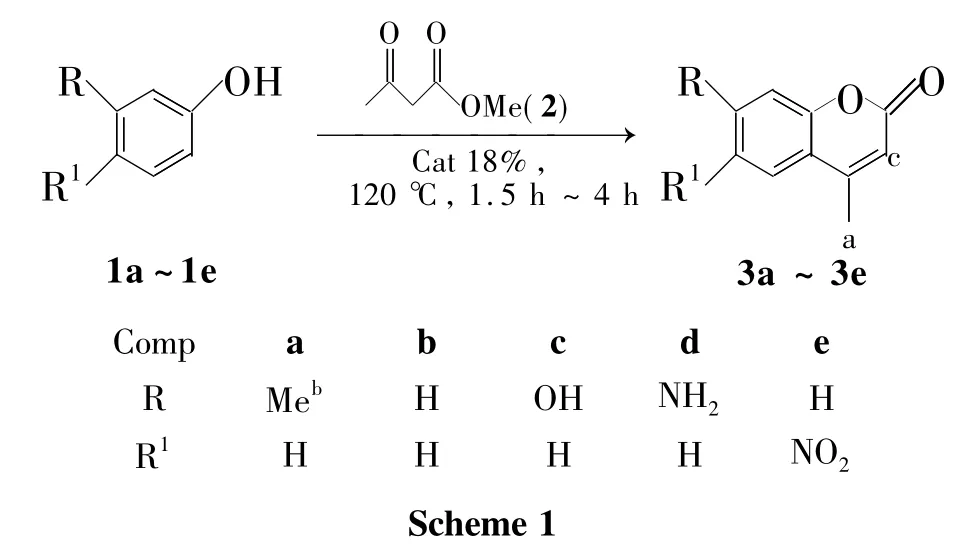
1 實驗部分
1.1 儀器與試劑
X-5型顯微熔點儀(溫度未校正);ZF1型三用紫外分析儀;Bruker AM-400型核磁共振儀(CDCl3為溶劑,TMS為內標);FT-IR-8400型傅里葉紅外光譜儀(KBr壓片);QP2010型氣相色譜-質譜聯用儀。
AC(Φ2.0 mm,碘值1 085 mg·g-1,CCl4質量分數94%),寧夏太西活性炭廠;其余所用試劑均為分析純。
1.2 合成
(1)Cat的合成[14]
在圓底燒瓶中加入濃硫酸200 mL,攪拌下加入AC 5.0 g,于室溫反應45 min。抽濾,濾餅用蒸餾水反復洗滌至中性,于120℃干燥得Cat,酸負載量1.84 mmol·g-1。
(2)3a~3e的合成(以3a為例)
在圓底燒瓶中依次加入間甲酚(1a)0.87 g(5 mmol),2 0.69 g(6 mmol)和Cat 0.96 g,攪拌下于120℃反應至終點(TLC跟蹤)。趁熱加入95%熱乙醇,過濾除去催化劑,濾液傾入25 mL冰水中,攪拌(析出大量固體),抽濾,濾餅用水洗滌后用乙醇重結晶得白色針狀晶體4,7-二甲基香豆素(3a)。
用類似方法合成白色晶體3b~3e。
3 a:m.p.130℃~131℃(131℃~132℃[15]);1H NMR δ:2.25(s,3H,a-H),2.33(s,3H,b-H),6.11(s,1H,c-H),6.59(s,1H,ArH),6.74(d,1H,ArH),7.40(d,1H,ArH); IR ν:1 552,1 623,1 705,3 062 cm-1。
4-甲基香豆素(3b):m.p.82℃~83℃(83℃~84℃[16]);1H NMR δ:2.32(s,3H,a-H),6.11(s,1H,c-H),6.61(d,1H,ArH),6.70(m,1H,ArH),6.81(m,1H,ArH),7.48(1H,d,ArH);IR ν:1 552,1 623,1 705,3 062 cm-1。
7-羥基-4-甲基香豆素(3c):m.p.185℃~187℃(183℃~184℃[17]);1H NMR δ:2.34(s,3H,a-H),6.10(s,1H,c-H),6.66(s,1H,ArH),6.74(d,J=8.58 Hz,1H,ArH),7.50 (d,J=8.58 Hz,1H,ArH),10.41(s,1H,OH);IR ν:3 495,3 111,1 667,1 608,1 456,845 cm-1。
7-氨基-4-甲基香豆素(3d):1H NMR δ:9.41 (s,1H,NH2),8.67(s,1H,c-H),7.55(1H,d,J=8.5 Hz,ArH),6.79(d,J=8.8 Hz,1H,ArH),6.76(s,J=2.3 Hz,1H,ArH),2.41(s,3H,a-H)。
7-硝基-4-甲基香豆素(3e):m.p.152℃~153℃(151℃~154℃[18]);1H NMR δ:2.32(s,3H,a-H),6.60(d,1H,ArH),6.72(m,1H,ArH),6.82(m,1H,ArH),7.49(d,1H,ArH),7.81(s,1H,c-H)。
2 結果與討論
2.1 反應條件優化
以1a和2反應合成3a為模板反應,分別考察原料配比[r=n(1a)∶n(2)]、反應溫度、催化劑用量及反應時間對反應的影響。
(1)r和反應溫度
1 a 5 mmol,其余反應條件同1.2(2),考察r和反應溫度對3a收率的影響,結果見表1。由表1可見,增加2用量和升高反應溫度均能提高3a收率;當r=1.0∶2.0,反應溫度為120℃時,收率最高(80%);繼續增大2用量和提高反應溫度,收率無明顯變化。因此,確定最佳r=1.0∶2.0,反應溫度為120℃。
(2)Cat用量和反應時間
1 a 5 mmol,r=1.0∶2.0,反應溫度為120℃,其余反應條件同1.2(2),考察Cat用量和反應時間對3a收率的影響,結果見表2。由表2可見,當Cat用量{[m(活性炭含酸的物質的量)/m (2)]×100%}為10%時,隨著反應時間從3 h (63%)增至5 h(70%),再增至7 h(71%),3a收率提高不明顯;當Cat用量為18%,反應3 h時收率最高(80%);繼續延長反應時間,收率基本不變,且略有降低。因此,確定Cat最佳用量18%,反應時間3 h。

表1 r和反應溫度對3a收率的影響*Table 1Effect of r and temperature on yield of 3a
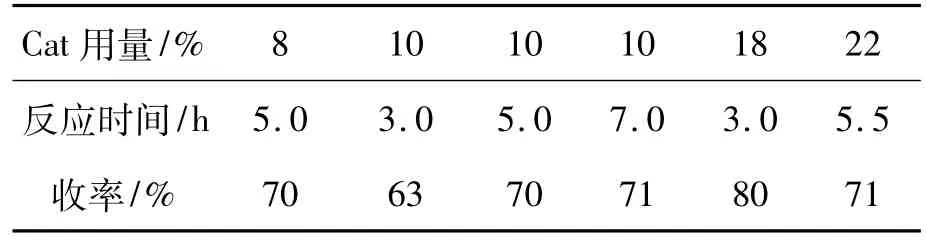
表2 Cat用量和反應時間對3a收率的影響*Table 2Effect of Cat dosage and time on yield of 3a

表3 底物結構對Pechmann反應的影響*Table 3Effect of substrate structure on Pechmann reaction
綜上所述,3a的最佳合成條件為:1a 5 mmol,r=1.0∶2.0,催化劑用量18%,于120℃反應3 h,收率80%。
2.2 底物結構對Pechmann反應的影響
考察底物1的結構對Pechmann反應的影響,結果見表3。由表3可見,一元酚、二元酚(1c)均可發生Pechmann香豆素環化反應,其中苯酚(1b)活性較低,收率58%;在苯酚苯環上引入強的供電子取代基,如氨基或羥基增強酚的反應活性,取代香豆素的收率較高;弱的給電子取代基甲基,對底物反應活性影響也較強;苯酚上含有吸電子取代基的底物(1e),反應活性減弱,收率僅10%,可能是硝基吸電子效應使得苯環上電子密度變小,導致酚的活性減弱。
3 結論
以濃硫酸改性活性炭為催化劑,取代酚與β-酮酯經Pechmann縮合反應合成了5個香豆素衍生物。實驗結果表明,在無溶劑條件下,催化劑用量18%,對取代基為供電子基的酚類,如羥基和氨基,具有較好的催化活性,收率在80%以上;而對取代基為吸電子基的酚類,收率很低,催化效果不理想。
該方法操作簡單、反應時間較短、收率較高、催化劑易制備且便宜、對環境污染極小。
[1]楊建明,呂劍.苯基磺酸官能化分子篩催化合成香豆素類化合物[J].有機化學,2004,24:450-453.
[2]Bahekara S S,Shinde D B.Samarium(Ⅲ)catalyzed one-pot construction of coumarins[J].Tetrahedron Lette,2004,45:7999-8001.
[3]Alexander V M,Bhat R P,Samant S D.Bismuth(Ⅲ)nitrate pentahydrate——a mild and inexpensive reagent for synthesis of coumarins under mild conditions[J].Tetrahedron Lett,2005,46:6957-6961.
[4]Muchchintala M,Vidavalur S,Guri L V D,et al.A solvent-free synthesis of coumarins via Pechmann condensation using heterogeneous catalyst[J].J Mol Catal A:Chem,2006,255:49-52.
[5]G Smitha,Ch Sanjeeva Reddy.ZrCl4-catalyzed pechmann reaction:Synthesis of coumarins under solventfree conditions[J].Synth Commun,2004,34(21): 3997-4003.
[6]H Valizadeha,A Shockravi.An efficient procedure for the synthesis of coumarin derivatives using TiCl4as catalyst under solvent-free conditions[J].Tetrahedron Letters,2005,46:3501-3503.
[7]D B Bose,A P Rudradas,M G Babu.The indium(Ⅲ)chloride-catalyzed von Pechmann reaction:Asimple and effective procedure for the synthesis of 4-substituted coumarins[J].Tetrahedron Letters,2002,43:9195-9197.
[8]Prajapati D,Gohain M.Iodine a simple,effective and inexpensive catalyst for the synthesis of substituted coumarins[J].Cata Lett,2007,119:59-63.
[9]B M Reddy,M K Patil,P Lakshmanan.Sulfated CexZr1-xO2solid acid catalyst for solvent free synthesis of coumarins[J].J Mol Catal A:Chem,2006,256:290-294.
[10]Yanlong Gu,Juan Zhang,Zhiying Duan,et al.Pechmann reaction in non-chloroaluminate acidic ionic liquids under solvent-free conditions[J].Adv Synth Catal,2005,347:512-516.
[11]Frère S,Thiéry V,Besson T.Microwave acceleration of the Pechmann reaction on graphite:montmorillonite K10:Application to the preparation of 4-substituted 7-aminocoumarins[J].Tetrahedron Letters,2001,42: 2791-2794.
[12]M S Manhas,S N Ganguly,S Mukherjee,et al.Microwave initiated reactions:Pechmann coumarin synthesis,Biginelli reaction,and acylation[J].Tetrahedron Lett,2006,47:2423-2425.
[13]Sachin B Patil,Ramakrishna P Bhat,Vivek P Raje,et al.Ultrasound-assisted pechmann condensation of phenols with β-ketoesters to form coumarins,in the presence of bismuth(Ⅲ)chloride catalyst[J].Synth Commun,2006,36:525-531.
[14]肖東彩,楊金會.超聲波促進濃H2SO4改性活性炭催化合成β-萘甲醚[J].精細化工,2010,27 (10):1038-1040.
[15]何懷國,侍愛秋,祁剛.香豆素及取代香豆素的合成[J].化工時刊,2007,21(9):34-35.
[16]Prajapati D,Gohain M.Iodine a simple,effective and inexpensive catalyst for the synthesis of substituted coumarins[J].Cata Lett,2007,119:59-63.
[17]吳純鑫.異黃酮類化合物合成新方法及3,4-二氫-2(1H)-喹啉酮衍生物合成新方法研究[D].杭州市:浙江大學,2004.
[18]王麗娟,董文亮,趙寶祥.Pechmann反應法制備香豆素的研究進展[J].合成化學,2007,15(3): 261-265.
Synthesis of Coumarin Derivatives Catalyzed by H2SO4/AC under Solvent-free Conditions
XIAO Dong-cai
(Yinchuan Energy College,Yinchuan 750105,China)
Five coumarin derivatives were synthesized by Pechmann condensation of substituted phenol and methyl acetoacetate(2)using active carbon(AC)supported concentrated sulfuric acid [H2SO4/AC(Cat)]as the catalyst under solvent-free conditions.The structures were confirmed by1H NMR,13C NMR and IR.The effects of raw material ratio,reaction temperature,reaction time and Cat dosage on Pechmann condensation were investigated by reaction of metacresyl(1a)with 2. The results showed that the optimum reaction conditions at 120℃for 3 h were as follows:1a 5 mmol,r=n(1a)∶n(2)=1.0∶2.0,Cat dosage was 18%,the yield of 3a was 80%.
H2SO4/AC;Pechmann condensation;solvent-free reaction;catalyst;synthesis
O625;O621.3
A
1005-1511(2014)03-0408-04
2013-03-21;
2014-04-16
肖東彩(1980-),女,寧夏銀川人,碩士研究生,講師,主要從事化工教學工作。E-mail:nxxdc@126.com

