125I粒子短時低劑量率照射對胰腺癌Capan-2細胞神經浸潤的影響
司佩任 路箏 劉巖 馬建霞 吳洪玉 李兆申
·論著·
125I粒子短時低劑量率照射對胰腺癌Capan-2細胞神經浸潤的影響
司佩任 路箏 劉巖 馬建霞 吳洪玉 李兆申
目的觀察125I粒子短時低劑量率照射對胰腺癌Capan-2細胞神經浸潤的影響,并探討其分子機制。方法建立胰腺癌Capan-2細胞和大鼠背根神經節(DRG)共培養及Capan-2或DRG單培養模型。通過125I粒子低劑量率照射平板對3種模型進行照射,以相應未照射模型作為對照。倒置顯微鏡下觀察癌細胞、DRG的生長,圖像分析軟件計算神經突和癌細胞集落占據的表面積,ELISA法檢測細胞培養上清液和基質膠溶解液中神經生長因子(NGF)及轉化生長因子α(TGF-α)濃度, RT-PCR法檢測胰腺癌Capan-2細胞神經營養因子-3 (NT-3)mRNA表達。結果共培養模型中DRG發出的神經突向癌細胞定向、集中生長,而癌細胞沿神經突的方向生長。經125I粒子照射后這種定向、集中和互逆的生長受到一定程度的抑制。共培養組第5天所增加的神經突表面積為290.15±12.08,較DRG單培養組的124.83±6.96顯著增加(P<0.01),經照射后的神經突表面積減少到201.53±12.20(P<0.01);所增加的Capan-2細胞表面積為300.47±12.99,較Capan-2細胞單培養組的199.30±8.60顯著增加(P<0.01),經照射后的Capan-2細胞表面積減少到202.35±7.97(P<0.01)。共培養組不表達NT-3 mRNA,經照射后NT-3mRNA表達量為0.68±0.04(P<0.05)。共培養組培養上清液中NGF及TGF-α濃度分別為(27.56±13.73)、(40.86±20.73)ng/ml,經照射后分別升高到(94.98±33.80)、(157.54±83.76)ng/ml,差異有統計學意義(P<0.05或<0.01)。共培養組基質膠溶解液中NGF及TGF-α濃度分別為(60.42±33.03)、(64.39±21.52)ng/ml,經照射后分別升高到(132.52±53.01)、(138.38±83.58)ng/ml,其中NGF的差異有統計學意義(P<0.05)。結論125I粒子短時低劑量率照射可以抑制胰腺癌和神經的交互作用,其機制可能與癌細胞促神經浸潤介質NGF、TGF-α和NT-3等表達上調有關。
胰腺腫瘤; 碘同位素; 小劑量照射; 腫瘤浸潤
胰腺癌遠處轉移擴散的途徑包括血管、淋巴管和神經浸潤,其中周圍神經浸潤(perineural invasion, PNI)是被廣泛接受的特殊擴散途徑[1]。極高頻率的PNI(90%,甚至100%)[1-3]是胰腺癌的特征。最初的胰腺內PNI可能引起胰腺外神經叢浸潤,是持續胰腺外播散和術后復發的主要原因[4]。最近的研究[5-7]證實,PNI可能涉及腫瘤細胞和神經元之間相互的趨向性和旁分泌的交互作用。
胰腺癌內放療治療是通過置入瘤內的放射性粒子持續釋放射線來達到最大限度地殺傷腫瘤細胞的作用。目前文獻報道[8-9],采用內鏡超聲(EUS)引導下穿刺等方式植入放射性粒子治療不能手術的中晚期胰腺癌是安全有效的,可明顯改善患者生活質量,尤其可以緩解患者的疼痛反應。胰腺癌疼痛的發生多與其嗜神經特性有關。為此,本研究用高神經轉移的胰腺癌細胞Capan-2和大鼠背根神經節(dorsal root ganglion,DRG)構建共培養體,觀察125I粒子短時低劑量照射對胰腺癌細胞、DRG生長及培養上清中PNI相關細胞因子含量的影響,探討胰腺癌細胞PNI的分子機制。
材料和方法
一、胰腺癌細胞神經浸潤的體外模型建立
4只SD雄性大鼠由第二軍醫大學實驗中心提供,動物許可證號SCXK(滬)2012-0003,清潔級,體質量80 g左右。參照文獻[5]用CO2將大鼠安樂死,75%乙醇消毒。在無菌條件下切除胸和腰段的DRGs,置RPMI培養液中備用。
胰腺癌細胞株Capan-2購自ATCC(American type cultrue collection),常規培養、傳代。參照文獻[5]把100 μl EHS基質膠(BD Biosciences公司)加進置于冰上(為保持基質膠的流動性)的直徑3.5 cm的細胞培養皿中央,放入1枚DRG,再種植105個Capan-2細胞,置細胞培養箱中加溫到37℃,使細胞和DRG固化在基質膠中。同時制作單獨種植Capan-2或DRG的培養皿。然后在培養皿中加入含有10%熱滅活的胎牛血清(GIBCO公司)、100 U/ml青霉素、100 μg/ml鏈霉素的RPMI-1640培養液(GIBCO公司)。
二、125I粒子短時低劑量率照射模型建立
參照文獻[10]設計。用5 cm直徑的細胞培養皿灌注石蠟,晾干后制作照射平板。在3 cm直徑的圓周線找出8個等距離的點,在這8個點和圓心處刻出直徑6 mm的凹槽。把9顆1.0 mCi的125I粒子分別置入9個凹槽內,制成照射平板。將上述制作的3種體外模型培養皿分別放在照射裝置的中央,置入專用的細胞培養箱中常規培養,總照射劑量為2 Gy。為避免各培養皿放射線的互相干擾,每個125I粒子照射裝置用金屬隔板隔開。以上述制作的3種體外模型培養皿放入普通培養箱內培養作為相應對照組。培養第3、5天在倒置顯微鏡下觀察各組Capan-2細胞、DRGs的形態并攝像,應用圖像分析軟件Image pro-plus 5.0計算DRG發出的神經突和選定區域的Capan-2細胞集落占據的表面積。照射第5天收集培養液上清,應用基質膠溶解劑(BD Biosciences公司)試劑盒將基質膠溶解并收集基質膠溶解液,收集從基質膠溶解液中分離出的Capan-2細胞和DRGs。實驗期間避免放射污染,凡是涉及接觸放射源的步驟均用鉛衣、鉛眼鏡、鉛圍脖和鉛手套防護。
三、人神經生長因子(NGF)、轉化生長因子α(TGF-α)檢測
應用NGF、TGF-α的ELISA試劑盒(Westang公司)檢測收集的細胞培養上清液和基質膠溶解液內NGF、TGF-α含量,按試劑盒說明書操作。通過試劑盒攜帶的NGF或TGF-α標準品繪制標準曲線,計算樣本中NGF、TGF-α濃度。
四、Capan-2細胞神經營養因子3(NT-3)mRNA表達的檢測
收集各組用基質溶解劑后分離出來的Capan-2細胞,用預冷的PBS洗滌3次,應用Trizol 試劑(Invitrogen公司)提取細胞總RNA。采用逆轉錄試劑盒(Takara公司)合成cDNA。RT反應條件:37℃ 15 min,85℃ 5 s。采用RT-PCP法檢測NT-3 mRNA的表達。引物通過Primer Premier 5.0軟件設計,NT-3引物上游為5′-AAGTCATCGGCCATCGACA-3′, 下游為5′-TCAGTGCTCGGACGTAGGTT-3′,擴增片段200 bp;內參GAPDH引物上游為5′-GCACCGTCAAGGCTGAGAAC-3′,下游為5′-ATGGTGGTGAAGACGCCAGT-3′,擴增片段142 bp。引物均由上海英俊生物工程技術有限公司合成。PCR反應條件: 94℃ 4 min,94℃ 45 s、58℃ 45 s、72℃ 45 s,35個循環,最后72℃延伸10 min。PCR擴增產物經瓊脂糖凝膠電泳分離,ImageJ軟件掃描,以目的條帶與內參條帶的灰度比值表示mRNA的表達量。每組實驗重復3次,取均值。
五、統計學分析
結 果
一、各組Capan-2細胞和神經突的生長狀況
在共培養模型可以觀察到DRG與Capan-2細胞之間定向、集中和互逆的生長,即DRG發出的神經突向癌細胞定向、集中生長,而癌細胞沿神經突的方向生長。經125I粒子持續低劑量率照射后這種定向、集中和互逆的生長雖仍存在,但其趨勢受到一定程度的抑制(圖1)。
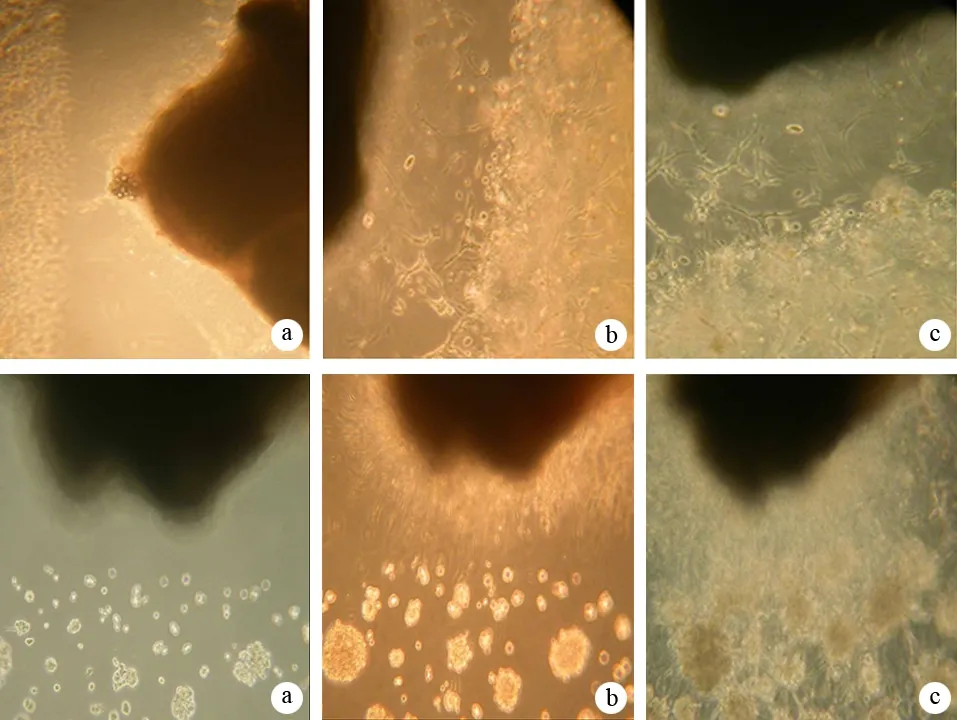
圖1共培養照射組(上)和未照射組(下)在第1(a)、3(b)、5(c)天的細胞和神經突生長
DRG和Capan-2共培養組在培養第3、5天所增加的神經突表面積分別為182.15±10.05、290.15±12.08,而DRG單培養組所增加的神經突表面積分別為82.08±3.48、124.83±6.96,共培養組顯著大于相應時間的DRG單培養組(t值分別為23.044、29.056,P值均<0.01)。經125I粒子照射后,共培養組所增加的神經突表面積分別為128.30±10.02、201.53±12.20,均顯著低于相應時間的共培養未照射組(t值分別為9.294、12.644,P值均<0.01)。
DRG和Capan-2共培養組在培養第3、5天所增加的Capan-2細胞表面積分別為162.93±6.96、300.47±12.99,而Capan-2細胞單培養組所增加的Capan-2細胞表面積分別為112.08±10.55、199.30±8.60,共培養組顯著大于相應時間的Capan-2細胞單培養組(t值分別為90.855、15.901,P值均<0.01)。經125I粒子照射后,共培養組所增加的Capan-2細胞表面積分別為95.12±4.83、202.35±7.97,均顯著低于相應時間的共培養未照射組(t值分別為19.610,16.116,P值均<0.01)。
二、各組Capan-2細胞NT-3 mRNA表達的變化
DRG和Capan-2共培養照射組NT-3 mRNA表達量為0.68±0.04,而共培養未照射組不表達NT-3 mRNA,差異有統計學意義(t=5.66,P<0.05)。Capan-2細胞單培養照射組NT-3 mRNA表達量為0.71±0.04,較單培養未照射組的0.57±0.03明顯上調,差異有統計學意義(Z=2.882,P<0.05,圖2)。

圖2Capan-2細胞單培養未照射組(1)和照射組(2)及共培養未照射組(3)和照射組(4) NT-3 mRNA的表達
三、各組Capan-2細胞培養上清和基質膠溶解液中NGF濃度的變化
DRG和Capan-2共培養照射組的培養上清中NGF濃度為(94.98±33.80)ng/ml,較共培養未照射組的(27.56±13.73)ng/ml顯著升高,差異有統計學意義(t=4.132,P<0.01)。Capan-2細胞單培養照射組的培養上清中NGF濃度為(24.62±10.10)ng/ml,單培養未照射組為(38.89±10.60)ng/ml,差異無統計學意義(t=2.182,P=0.06)。
DRG和Capan-2共培養照射組的基質膠溶解液中NGF濃度為(132.52±53.01)ng/ml,較共培養未照射組的(60.42±33.03)ng/ml顯著升高,差異有統計學意義(t=2.581,P<0.05)。Capan-2細胞單培養照射組的基質膠溶解液中NGF濃度為(56.59±8.15)ng/ml,單培養未照射組為(57.81±3.12)ng/ml,差異無統計學意義(t=0.410,P=0.890)。
四、各組Capan-2細胞培養上清和基質膠溶解液中TGF-α濃度的變化
DRG和Capan-2共培養照射組的培養上清中TGF-α濃度為(157.54±83.76)ng/ml,較共培養未照射組的(40.86±20.73)ng/ml顯著升高,差異有統計學意義(t=2.704,P<0.05)。Capan-2細胞單培養照射組培養上清中TGF-α濃度為(54.80±34.71)ng/ml,單培養未照射組為(105.21±21.97)ng/ml,差異無統計學意義(t=2.454,P=0.05)。
DRG和Capan-2共培養照射組基質膠溶解液中TGF-α濃度為(138.38±83.58)ng/ml,共培養未照射組為(64.39±21.52)ng/ml,差異無統計學意義(t=1.917,P=0.09)。Capan-2細胞單培養照射組基質膠溶解液中TGF-α濃度為(157.71±39.57)ng/ml,較單培養未照射組的(83.39±26.69)ng/ml顯著升高,差異有統計學意義(t=3.359,P<0.05)。
討 論
以前的研究多用前列腺癌細胞與DRG共培養證實腫瘤細胞沿神經節起源的神經突移行,神經突集中、定向性向腫瘤細胞集落外生長[5]。同樣,胰腺癌細胞與DRG共培養模型中,腫瘤細胞在移行前經歷早期形態學改變和神經細胞的神經突定向朝向癌細胞生長,最終導致移行細胞成簇圍繞在神經節周圍[6-7]。這些發現提示神經元與癌細胞之間存在互惠的交互作用,這種交互作用有助于腫瘤細胞的增殖和抑制凋亡,并有利于神經生長。
神經營養因子家族(NTs)包括NGF、NT-3等,在胰腺癌的發生和演進過程中發揮重要作用,包括刺激趨化性、腫瘤侵襲性、克隆增生、各種腫瘤細胞的形態學改變等[4]。
NGF在胰腺癌細胞系中過表達,其受體trkA在周圍神經的神經束膜上強表達,NGF與trkA相互作用調節神經浸潤[6]。NT-3在胰腺癌標本中過表達,刺激腫瘤細胞浸潤[11]。表皮生長因子受體(EGFR)和TGF-α在有胰腺癌細胞的組織中大量表達,神經也產生TGF-α,TGF-α和EGFR相互作用觸發一系列增強細胞增殖的事件,與胰腺癌的神經浸潤有關[12]。
針對胰腺癌細胞與神經節之間互惠的交互作用,以及這些與胰腺癌神經浸潤有關的因子,本研究結合文獻資料[5,10]構建了一個新的125I籽源短時間低劑量率照射干預胰腺癌神經浸潤的體外模型,用125I籽源短時間低劑量率持續照射(總照射劑量為2 Gy)的方法干預胰腺癌細胞與神經的交互作用,觀察癌細胞與神經突的生長和形態變化, 以及NGF、NT-3和TGF-α表達的改變。結果發現,125I籽源短時間低劑量率持續照射后在形態學和生物學行為上抑制胰腺癌細胞和神經之間的交互作用,使癌細胞向神經突的生長和移行減緩。但在共培養模型培養上清液和基質膠溶解液中,照射組的NGF和TGF-α濃度高于未照射對照組,且照射組胰腺癌細胞NT-3 mRNA的表達顯著上調,提示125I籽源短時間低劑量率持續照射可能增強胰腺癌細胞的侵襲性,與Ohuchid等[13]的研究一致。他們把照射過的成纖維細胞與胰腺癌細胞共培養,可刺激成纖維細胞分泌MMP-2,增強腫瘤細胞的侵襲力,但使胰腺癌細胞的移行能力減弱,這也可以解釋本研究在體外模型中看到的現象,即神經節與癌細胞的互逆生長明顯減弱。
以前的研究認為,放療可作為很多惡性腫瘤主要的一種輔助治療。應用于胰腺癌患者放療的原理是基于放射能抑制體外細胞增殖或使凋亡的細胞死亡,并抑制體內腫瘤的生長[14]。然而,近來很多證據顯示放射通過激活與腫瘤浸潤和轉移有關的眾多途徑促進癌細胞的惡性行為[15-17]。此現象可能由于多為體外實驗,與體內腫瘤生長的微環境不同,導致對照射的反應不同。其次實驗多為短時低劑量率照射,照射時間及劑量是否充分也是值得考慮的問題。因此深入研究125I粒子短時低劑量率照射干預胰腺癌的分子機制顯得尤為重要。
[1] Kayahara M, Nakagawara H, Kitagawa H, et al. The Nature of Neural Invasion by Pancreatic Cancer. Pancreas, 2007, 35:218-223.
[2] Spinelli GP, Zullo A, Romiti A, et al.Long-term survival in metastatic pancreatic cancer. A case report and review of the literature.JOP, 2006, 7:486-491.
[3] Beard CJ, Chen MH, Cote K, et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys, 2004, 58: 19-24.
[4] Liebig C, Ayala G, Wilks JA,et al. Perineural invasion in cancer: a review of the literature. Cancer, 2009,115:3379-3391.
[5] Ayala GE, Wheeler TM, Shine HD, et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate, 2001, 49: 213-223.
[6] Dai H, Li R, Wheeler T, et al. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol, 2007, 38:299-307.
[7] Ceyhan GO, Demir IE, Altintas B, et al. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun, 2008, 37:442-447.
[8] Jin Z, Du Y, Li Z, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125 seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective study, Endoscopy, 2008, 40:314-320.
[9] Wang K, Jin Z, Du Y, et al. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: a prospective pilot study. Gastrointest Endosc, 2012, 76:945-952.
[10] Aird EG,Folkard M,Mayes CR,et al.A purpose-built iodine 125 irradiation plaque for low dose rate low energy irradiation of celllines in vitro. Br J Radiol, 2001,74:56-61.
[11] Okada Y, Eibl G, Guha S, et al. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis, 2004, 21: 285-292.
[12] Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res, 2008,149:319-328.
[13] Ohuchida K, Mizumoto K, Murakami M, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions.Cancer Res, 2004, 64:3215-3222.
[14] Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett, 2001, 169:107-114.
[15] Sakamoto Y, Kitajima Y, Edakuni G, et al. Expression of Trk tyrosine kinase receptor is a biologic marker for cell proliferation and perineural invasion of human pancreatic ductal adenocarcinoma. Oncol Rep, 2001, 8:477-484.
[16] Bouzas-Rodriguez J,Cabrera JR, Delloye-Bourgeois C,et al.Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis.J Clin Invest, 2010, 120:850-858.
[17] Meirovitz A, Hermano E, Lerner I, et al. Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma. Cancer Res, 2011,71:2772-2780.
EffectofIodine125seedsshorttimelowdoserateirradiationonperineuralinvasioninpancreaticcancerCapan-2cells
SIPei-ren,LUZheng,LIUYan,MAJian-xia,WUHong-yu,LIZhao-shen.
DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
LIZhao-shen,Email:zhsli@81890.net
ObjectiveTo investigate the effect of Iodine 125 seeds short time low dose rate irradiation on perineural invasion (PNI) in pancreatic cancer Capan-2 cells, and explore its molecular mechanism.MethodsThe co-culture model was established by co-culturing the dorsal root ganglion (DRG) of SD rat and Capzn-2 cells line , while Capan-2 culture model and DRG culture model was also established. Iodine 125 seeds short time low dose rate irradiation tablet was used for the 3 models, and the model without irradiation was used as control. Cancer cell and DRG growth was observed under inverted microscopy, surface of neurite and cell colony growth was determined by image analysis software. The concentration of nerve growth factor (NGF), transforming growth factor-α (TGF-α) in cell culture supernatant and matrigel solution was tested by ELISA, and the expression of neurotrophin-3 (NT-3) mRNA was detected by semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR).ResultsIn the co-culture model, neurite of DRG showed a direction to cancer cells and had a concentrated growth towards cancer cells. And Capan-2 cells formed more colonies towards neurite. However, in irradiation groups, the symbiotic phenomenon was inhibited to some degree. Increased surface of neurite in co-culture model at 5th day was 290.15±12.08, which was significantly higher than that in DRG group (124.83±6.96,P<0.01), but the surface of neurite was decreased to 201.53±12.20 after irradiation (P<0.01). Increased surface of Capan-2 cell was 300.47±12.99, which was significantly higher than that in Capan-2 group (199.30±8.60,P<0.01 ), but the surface of Capan-2 was decreased to 202.35±7.97 after irradiation (P<0.01). NT-3 mRNA was seldom or not expressed in supernatant of co-culture model, but it was strongly expressed (0.68±0.04) after irradiation (P<0.05). The concentration of NGF and TGF-α in supernatant of co-culture model were (27.56±13.73), (40.86±20.73)ng/ml, after irradiation they were increased to (94.98±33.80), (157.54±83.76)ng/ml, and the difference between the two groups was statistically significant (P<0.05 or <0.01). The concentration of NGF and TGF-α in matrigel lysate of co-culture model were (60.42±33.03), (64.39±21.52)ng/ml, after irradiation they were increased to (132.52±53.01), (138.38±83.58)ng/ml, and the difference of NGF concentration between the two groups was statistically significant (P<0.05).ConclusionsIodine-125 seeds short-time low-dose rate irradiation could inhibit interactions between nerve and Capan-2 cells, and the mechanism may be related to up-regulation of cancer cells perineural invasion promoter NGF, TGF-α and NT-3.
Pancreatic neoplasms; Iodine isotopes; Low-level irradiation; Neoplasm invasiveness
2013-07-10)
(本文編輯:屠振興)
10.3760/cma.j.issn.1674-1935.2013.06.001
國家自然科學青年基金(30801362)
264002 山東煙臺,解放軍107醫院(司佩任);第二軍醫大學長海醫院消化內科(路箏、劉巖、馬建霞、吳洪玉、李兆申)
李兆申,Email:zhsli@81890.net
共同第一作者:吳洪玉

