替米沙坦對大鼠腦缺血后核因子κB表達的影響
祝春華,溫 雅,王力娜,馬悅霞
(河北醫(yī)科大學(xué)第二醫(yī)院神經(jīng)內(nèi)科,河北石家莊050000)
·論 著·
替米沙坦對大鼠腦缺血后核因子κB表達的影響
祝春華,溫 雅,王力娜,馬悅霞
(河北醫(yī)科大學(xué)第二醫(yī)院神經(jīng)內(nèi)科,河北石家莊050000)
目的探討抗高血壓藥物替米沙坦對大鼠腦梗死的治療作用及其機制。方法線栓法建立大腦中動脈閉塞(middle cerebral artery occlusion,MCAO)大鼠模型。替米沙坦治療后,觀察其神經(jīng)功能評分、腦梗死體積和患側(cè)腦水腫,采用免疫組織化學(xué)法、Western blot和實時熒光定量聚合酶鏈反應(yīng)檢測核因子κB(nuclear factorκB,NF-κB)和過氧化物酶體增殖物激活受體γ(peroxisome proliferator-activated receptors,PPARγ)基因和蛋白變化。結(jié)果MCAO后24 h,與假手術(shù)組比較,替米沙坦能明顯改善神經(jīng)功能評分、減輕腦水腫、減小梗死體積,同時顯著下調(diào)NF-κB,上調(diào)PPARγ的蛋白表達和基因表達;與GW9662(特異性阻斷劑)聯(lián)合干預(yù),上述作用消失。結(jié)論替米沙坦能保護腦缺血性腦組織,其作用機制可能是激活PPARγ、抑制NF-κB表達。
腦缺血;NF-κB;過氧化物酶體增殖物激活受體
替米沙坦(telmisartan)是臨床常用的血管緊張素受體阻斷劑類抗高血壓藥物。高血壓病是腦血管病患者的主要危險因素,近年研究[1-2]發(fā)現(xiàn),替米沙坦不僅通過調(diào)控血壓保護重要的靶器官——腦組織,還對腦缺血具有良好的抗炎和腦保護作用,能夠顯著減輕腦梗死體積、腦水腫和繼發(fā)性腦損傷,其機制多與過氧化物酶體增殖物激活受體γ(peroxisome proliferator-activated receptors,PPARγ)有關(guān)。如果替米沙坦在調(diào)整血壓的同時,對腦梗死組織有保護作用,達到一舉兩得的效果,將有利于臨床治療。核因子κB(nuclear factorκB,NF-κB)在介導(dǎo)神經(jīng)細胞凋亡、炎癥反應(yīng)等方面的作用至關(guān)重要[3-7]。因此,本研究以大鼠大腦中動脈閉塞(middle cerebral artery occlusion,MCAO)模型為研究對象,以替米沙坦進行
干預(yù)治療,通過觀察替米沙坦對腦梗死大鼠的神經(jīng)功能評分、減輕腦水腫、減小梗死體積以及NF-κB和PPARγ表達的影響,探討替米沙坦對腦梗死治療作用及機制。
1 資料與方法
1.1 動物與試劑
1.1.1 實驗動物:成年健康雄性清潔級SD大鼠90只,體質(zhì)量250~280g,由河北省實驗動物中心提供。
1.1.2 試劑和儀器:兔抗PPARγ多克隆抗體(美國Santa Craz);兔抗NF-κBp65多克隆抗體(美國Santa Craz);替米沙坦(德國Boehringer-Ingelheim);GW9662(美國Sigma);DYY-12型電泳儀(北京六一儀器廠);Synergy-HT多功能酶標儀(美國Bio-Tek);PCR擴增儀(德國Eppendorf);熒光定量PCR儀(7500)(美國ABI)。
1.1.3 引物設(shè)計與合成:上海生物工程公司合成,引物序列如下,PPARγ,上游5′-GAAGACATCCCGTTCACAAGA-3′,下游5′-TGAT GCTTTATCCCCACAGAC-3′,擴增全長207 bp;NF-κB,上游5′-AGCTCCTGTCCCAGTTCTAGC-3′,下游5′-ACTCCTGGGTCTGTGTTGTTG-3′,擴增全長168bp;β-actin,上游5′-GGAGATTACTGCCCTGGCTCC-TA-3′,下游5′-GACTCATCGTACTCCTGCTTGCTG-3′,擴增全長150bp。
1.2 實驗方法
1.2.1 動物分組和模型:隨機將90只SD大鼠分為假手術(shù)組、溶劑對照組、替米沙坦組、替米沙坦+GW9662組。采用本實驗室成熟的線栓法建立持續(xù)性大鼠右側(cè)MCAO模型[3-4]。替米沙坦組30mg/kg于MCAO手術(shù)前1h灌胃給藥;假手術(shù)組和對照組給予等體積生理鹽水;替米沙坦+GW9662組于MCAO手術(shù)前1h以灌胃方式給予30mg/kg替米沙坦,術(shù)后立即腹腔注射4mg/kg GW9662。
1.2.2 神經(jīng)功能缺失評分:將各組大鼠分別于相應(yīng)時間點進行神經(jīng)功能評分后斷頭處死,采用改良的Longa分級法單盲進行行為學(xué)評分。0分,無缺陷;1級,不能伸展對側(cè)前肢;2分,對側(cè)前肢屈曲;3分,輕度向?qū)?cè)轉(zhuǎn)圈;4分,嚴重向?qū)?cè)轉(zhuǎn)圈;5分,向?qū)?cè)跌倒(n=6)。
1.2.3 腦組織含水量測定:在相應(yīng)時間點斷頭取腦,冠狀切開去除額極,雙側(cè)取約2mm厚的腦組織,采用干濕法測定腦含水量[(腦組織濕質(zhì)量-腦組織干質(zhì)量)/濕質(zhì)量×100%](n=8)。
1.2.4 腦梗死體積測定:在相應(yīng)時間斷頭取腦,均勻切成5片冠狀切片,浸入2%TTC溶液,37℃染色30min后,4%多聚甲醛中固定24h。梗死體積百分比=(矯正梗死體積/非缺血側(cè)半球體積)×100%(n=6)。
1.2.5 NF-κB和PPARγ蛋白和基因表達:采用免疫組織化學(xué)法(n=3)和Western blotting(n=4)檢測NF-κB和PPARγ蛋白表達。采用實時熒光定量PCR(n=3)檢測NF-κB和PPARγmRNA表達。
1.3 統(tǒng)計學(xué)方法:應(yīng)用SPSS11.0統(tǒng)計軟件進行數(shù)據(jù)分析,計量資料以±s表示,多組數(shù)據(jù)比較采用單因素方差分析。P<0.05為差異有統(tǒng)計學(xué)意義。
2 結(jié) 果
2.1 替米沙坦改善大鼠MCAO后的神經(jīng)功能:溶劑對照組與假手術(shù)組比較,MCAO后有明顯的神經(jīng)功能障礙。替米沙坦組與溶劑對照組和替米沙坦+GW9662組比較,神經(jīng)功能障礙明顯改善(P<0.05);替米沙坦+GW9662組與溶劑對照組比較差異有統(tǒng)計學(xué)意義。見表1。
表1 各實驗組術(shù)后24h神經(jīng)功能缺失評分比較Table 1 Neurological deficits at 24h after operation in different experimental groups ±s,scores)
*P<0.05 vs Sham group #P<0.05 vs vehicle group and telmisartan+GW9662 group by q test
Groups Neurological deficits Sham 0 Vehicle 4.00±0.89*Telmisartan 2.50±1.05*#Telmisartan+GW9662 3.83±0.75*
2.2 替米沙坦顯著減輕患側(cè)腦水腫:術(shù)后24h,與假手術(shù)組比較,其余3組患側(cè)腦組織有明顯的水腫(P<0.05);替米沙坦組與溶劑對照組和替米沙坦+GW9662組比較,患側(cè)腦水腫明顯減輕(P<0.05)。4組大鼠對側(cè)腦組織含水量差異無統(tǒng)計學(xué)意義。見表2。
表2 各實驗組術(shù)后24h腦組織含水量比較Table 2 Brain water content of ipsilateral and contrallateral hem isphere at 24h after operation in different experimental groups ±s,%)

表2 各實驗組術(shù)后24h腦組織含水量比較Table 2 Brain water content of ipsilateral and contrallateral hem isphere at 24h after operation in different experimental groups ±s,%)
*P<0.05 vs sham group #P<0.05 vs vehicle group and telmisartan+GW9662 group by q test
Groups Brain water content Ipsilateral Contralateral 0.788 4±0.005 4 Sham 0.785 1±0.005 0 0.784 6±0.005 5 Vehicle 0.854 0±0.005 5*0.785 6±0.002 6 Telmisartan 0.819 1±0.008 6*#0.788 1±0.006 0 Telmisartan+GW9662 0.851 1±0.008 1*
2.3 替米沙坦顯著減小腦梗死體積:假手術(shù)組未見缺血病灶,溶劑對照組、替米沙坦組和替米沙坦+GW9662組均有大小不等的缺血性病灶(P<0.05)。替米沙坦組與溶劑對照組和替米沙坦+GW9662組比較,缺血性病灶體積明顯減小(P<0.05)。見表3。
表3 各實驗組術(shù)后24h腦梗死體積比較Table 3 Infarct volume at 24h after operation in different experimental groups ±s,%)
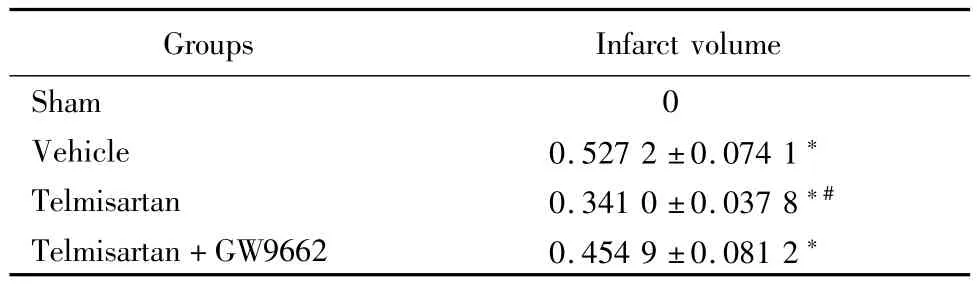
表3 各實驗組術(shù)后24h腦梗死體積比較Table 3 Infarct volume at 24h after operation in different experimental groups ±s,%)
*P<0.05 vs sham group #P<0.05 vs vehicle group and telmisartan+GW9662 group by q test
Groups Infarct volume Sham 0 Vehicle 0.527 2±0.074 1*Telmisartan 0.341 0±0.037 8*#Telmisartan+GW9662 0.454 9±0.081 2*
2.4 替米沙坦調(diào)節(jié)NF-κB蛋白和基因表達:免疫組織化學(xué)法顯示,與假手術(shù)組比較,溶劑對照組和替米沙坦+GW9662組PPARγ陽性細胞數(shù)明顯減少(P<0.05),NF-κB陽性細胞數(shù)明顯增多(P<0.05);替米沙坦組與溶劑對照組和替米沙坦+GW9662組比較,PPARγ陽性細胞數(shù)明顯增多(P<0.05),NF-κB陽性細胞數(shù)明顯減少(P<0.05)。見表4。
Western blot和實時定量PCR結(jié)果顯示,與假手術(shù)組比較,溶劑對照組和替米沙坦+GW9662組NF-κB蛋白以及基因表達明顯上調(diào)(P<0.05),PPARγ表達明顯下降;替米沙坦組與溶劑對照組和替米沙坦+GW9662組比較,NF-κB蛋白以及基因表達明顯下調(diào),PPARγ表達明顯上調(diào)(P<0.05)。見表5,6。
表4 替米沙坦調(diào)節(jié)NF-κB和PPARγ表達陽性細胞數(shù)Table 4 Telm isartan regulates NF-κB and PPARγpositive cell number ±s,%)
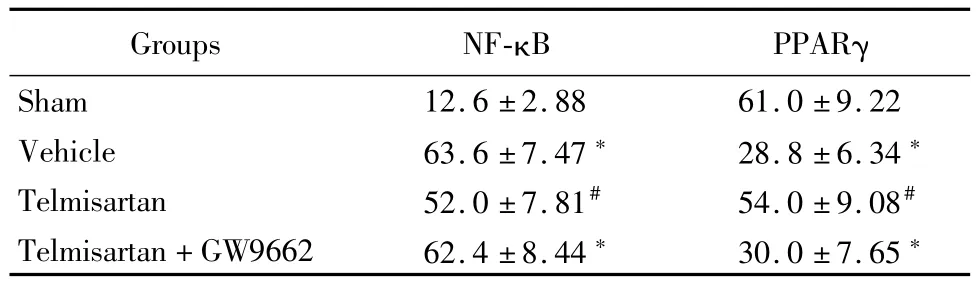
表4 替米沙坦調(diào)節(jié)NF-κB和PPARγ表達陽性細胞數(shù)Table 4 Telm isartan regulates NF-κB and PPARγpositive cell number ±s,%)
*P<0.05 vs sham group #P<0.05 vs vehicle group and telmisartan+GW9662 group by q testPPARγ:peroxisome proliferator-activated receptors;NF-κB:nuclear factor κB
Groups NF-κB PPARγ Sham 12.6±2.88 61.0±9.22 Vehicle 63.6±7.47*28.8±6.34*Telmisartan 52.0±7.81#54.0±9.08#Telmisartan+GW9662 62.4±8.44*30.0±7.65*
表5 替米沙坦調(diào)節(jié)NF-κB和PPARγ蛋白表達Table 5 Telm isartan regulates NF-κB and PPARγprotein expression ±s,%)
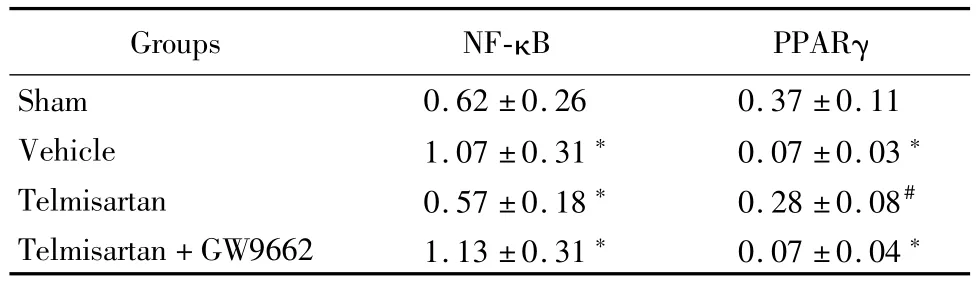
表5 替米沙坦調(diào)節(jié)NF-κB和PPARγ蛋白表達Table 5 Telm isartan regulates NF-κB and PPARγprotein expression ±s,%)
*P<0.05 vs sham group #P<0.05 vs vehicle group and telmisartan+GW9662 group by q testPPARγ:peroxisome proliferator-activated receptors;NF-κB:nuclear factor κB
Groups NF-κB PPARγ Sham 0.62±0.26 0.37±0.11 Vehicle 1.07±0.31*0.07±0.03*Telmisartan 0.57±0.18*0.28±0.08#Telmisartan+GW9662 1.13±0.31*0.07±0.04*
表6 替米沙坦調(diào)節(jié)NF-κB和PPARγ基因表達Table 6 Telm isartan regulates NF-κB and PPARγgene expression ±s,%)
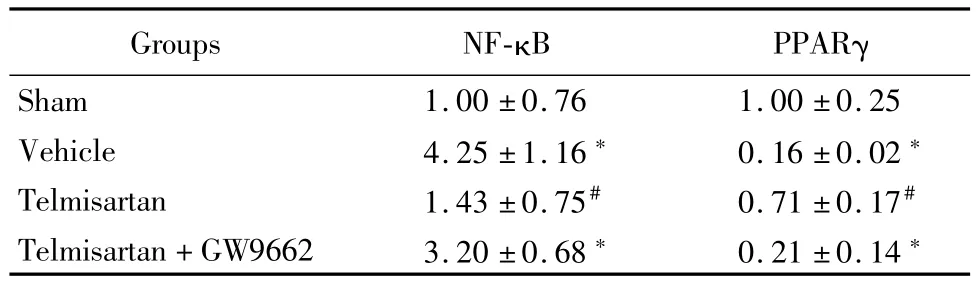
表6 替米沙坦調(diào)節(jié)NF-κB和PPARγ基因表達Table 6 Telm isartan regulates NF-κB and PPARγgene expression ±s,%)
*P<0.05 vs sham group #P<0.05 vs vehicle group and telmisartan+GW9662 group by q testPPARγ:peroxisome proliferator-activated receptors;NF-κB:nuclear factor κB
Groups NF-κB PPARγ Sham 1.00±0.76 1.00±0.25 Vehicle 4.25±1.16*0.16±0.02*Telmisartan 1.43±0.75#0.71±0.17#Telmisartan+GW9662 3.20±0.68*0.21±0.14*
3 討 論
NF-κB是腦缺血后啟動炎癥反應(yīng)的關(guān)鍵因子,其中p65是NF-κB在腦組織內(nèi)的主要活性亞基之一。正常情況下,NF-κB與κB抑制因子(I-κB)結(jié)合以非活性形式存在于細胞漿,在缺血缺氧刺激下,NF-κB與I-κB分離而激活后,向細胞核內(nèi)轉(zhuǎn)位,調(diào)節(jié)下游白細胞介素8、血管細胞黏附分子1、腫瘤壞死因子α等炎癥介質(zhì)的表達。同時,這些炎癥反應(yīng)的產(chǎn)物又再次激活NF-κB,使炎癥反應(yīng)形成持續(xù)放大的態(tài)勢[3,8-9]。有研究[10-11]發(fā)現(xiàn),NF-κB基因敲除能夠顯著減小腦梗死體積和炎癥反應(yīng)。進一步證明NF-κB是腦缺血后炎癥反應(yīng)和氧化應(yīng)激過程的重要參與者。
PPARγ是關(guān)鍵性核轉(zhuǎn)錄因子,活化后表現(xiàn)出抗炎、抗氧化、神經(jīng)保護等獨特優(yōu)勢,在保護缺血腦組織方面發(fā)揮的作用突出而得到越來越多的關(guān)注。研究發(fā)現(xiàn),PPARγ有效調(diào)節(jié)下游炎癥因子、抑制炎癥反應(yīng)與抑制NF-κB有關(guān),具體機制包括抑制I-κB分解、p65亞基向細胞核內(nèi)轉(zhuǎn)位以及與特定DNA序列的結(jié)合等。Remels等[12]在骨骼肌細胞中證實PPARγ的抗炎癥反應(yīng)通過抑制NF-κB活性發(fā)揮作
用。Zhang等[13]通過腦缺血的研究發(fā)現(xiàn),GW9662成功阻斷了吡格列酮抑制NF-κBp65活性的抗炎癥反應(yīng)作用,進一步證實PPARγ是NF-κB的上游調(diào)控基因。
PPARγ以結(jié)合配體的形式活化,目前確定的作用最強的PPARγ天然性配體是15d-PGJ2,最常見的合成性配體是噻唑烷二酮類化合物(thiazoli dinediones,TZDs)。腦缺血后PPARγ的DNA結(jié)合能力顯著下降,而激活劑包括15d-PGJ2和TZDs,如羅格列酮能夠全面恢復(fù)PPARγ的DNA結(jié)合能力,是其發(fā)揮抗炎癥反應(yīng)等作用的關(guān)鍵機制[14-16]。
替米沙坦不僅通過阻斷血管緊張素Ⅱ有效調(diào)控血壓,保護缺血腦組織,而且越來越多的研究發(fā)現(xiàn)其表現(xiàn)出顯著的抗炎癥反應(yīng)和氧化應(yīng)激的特點,是惟一具有PPARγ活化作用的血管緊張素Ⅱ受體拮抗劑類藥物。Maejima等[17]證實,替米沙坦通過激活PPARγ途徑抑制心肌梗死后基質(zhì)金屬蛋白酶9等炎癥因子表達,改善心室重構(gòu)。更多的腦缺血的研究已經(jīng)證實,替米沙坦能夠顯著減小腦缺血體積,減輕腦缺血后炎癥反應(yīng)[1-2]。本實驗結(jié)果與之一致,替米沙坦能夠顯著改善神經(jīng)功能缺失、減輕腦水腫和減小腦梗死體積,并有效下調(diào)腦缺血后高表達的NF-κB,上調(diào)PPARγ,而與PPARγ特異性阻斷劑聯(lián)合干預(yù)使替米沙坦的保護作用以及對NF-κB表達的抑制作用消失。因此,替米沙坦通過激活PPARγ抑制NF-κB的活性和表達,是其發(fā)揮抗炎癥反應(yīng)和腦保護作用的重要機制。
[1]HARAGUCHIT,IWASAKIK,TAKASAKIK,et al.Telmisartan,a partial agonist of peroxisome proliferator-activated receptor gamma,improves impairment of spatialmemory and hippocampal apoptosis in rats treated with repeated cerebral ischemia[J]. Brain Res,2010,1353(24):125-132.
[2]KOBAYASHIT,KAWAMATA T,SHIBATA N,et al.AngiotensinⅡtype 1 receptor blocker telmisartan reduces cerebral infarct volume and peri-infarct cytosolic phospholipase A(2)level in experimental stroke[J].JNeurotrauma,2009,26(12):2355-2364.
[3]LIU Y,ZHANG XJ,YANG CH,et al.Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-kappaB expression[J].Brain Research,2009,1268:174-180.
[4]YANG C,ZHANG X,F(xiàn)AN H,et al.Curcumin upregulates transcription factor Nrf2,HO-1 expression and protects rat brains against focal ischemia[J].Brain Research,2009,1282(28):133-141.
[5]WANG L,ZHANG X,LIU L,etal.Atorvastatin protects ratbrains against permanent focal ischemia and downregulates HMGB1, HMGB1 receptors(RAGE and TLR4),NF-kappaB expression[J].Neuroscience Letters,2010,471(3):152-156.
[6]WANG L,ZHANG X,LIU L,et al.TanshinoneⅡA downregulates HMGB1,RAGE,TLR4,NF-kappaB expression,ameliorates BBB permeability and endothelial cell function,and protects rat brains against focal ischemia[J].Brain Research,2010,1321(19):143-151.
[7]CUIL,ZHANG X,YANG R,et al.Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia:down-regulated 12/15-LOX,p38MAPK and cPLA2 expression,ameliorated BBB permeability[J].Brain Research,2010,1325(14):164-173.
[8]MALEK R,BOROWICZ KK,JARGIELLO M,et al.Role of NF-kappaB in the central nervous system[J].Pharmacol,2007,59(1):25-33.
[9]MATTSON MP,CAMANDOLAS.NF-kappaB in neuronal plasticity and neurodegenerative disorders[J].J Clin Invest,2001,107(3):247-254.
[10]SCHNEIDER A,MARTIN-VILLALBA A,WEIH F,et al.NF-kappaB is activated and promotes cell death in focal cerebral ischemia[J].Nat Med,1999,5(5):554-559.
[11]STEPHENSON D,YIN T,SMALSTIG EB,et al.Transcription factor nuclear factor-kappaB is activated in neurons after focal cerebral ischemia[J].JCereb Blood Flow Metab,2000,20(3):592-603.
[12]REMELS AH,LANGEN RC,GOSKER HR,et al.PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletalmuscle[J].Am JPhysiol Endocrinol Metab,2009,297(1):E174-183.
[13]ZHANG HL,XU M,WEI C,et al.Neuroprotective effects of pioglitazone in a ratmodel of permanent focal cerebral ischemia are associated with peroxisome proliferator-activated receptor gamma-mediated suppression of nuclear factor-kappaB signaling pathway[J].Neuroscience,2011,176(10):381-395.
[14]VICTOR NA,WANDERIEW,GAMBOA J.Altered PPARgamma expression and activation after transient focal ischemia in rats[J].Eur JNeurosci,2006,24(6):1653-1663.
[15]OU Z,ZHAO X,LABICHE LA,et al.Neuronal expression of peroxisome proliferator-activated receptor-gamma(PPARgamma)and 15d-prostaglandin J2--mediated protection of brain after experimental cerebral ischemia in rat[J].Brain Res,2006,1096(1):196-203.
[16]ZHAO Y,PATZER A,HERDEGEN T,et al.Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats[J].FASEB J,2006,20(8):162-75.
[17]MAEJIMA Y,OKADA H,HARAGUCHIG,et al.Telmisartan,a unique ARB,improves left ventricular remodeling of infracted heart by activating PPARgamma[J].Lab Invest,2011,91(6):932-944.
(本文編輯:劉斯靜)
THERAPEUTIC EFFECT AND MECHANISMSOF TELM ISARTAN ON FOCAL CEREBRAL ISCHEM IA IN RATS
ZHU Chunhua,WEN Ya,WANG Lina,MA Yuexia
(Department of Neurology,the Second Hospital of HebeiMedical University,Shijiazhuang 050000,China)
ObjectiveTo explore the therapeutic effect and mechanisms of telmisartan on focal cerebral ischemia of rats.M ethodsMale Sprague-Dawley rats were subjected to permanent middle cerebral artery occlusion(MCAO).Telmisartan was systemically administered to explore the effect on nuclear factorκB(NF-κB)and peroxisome proliferator-activated receptors(PPARγ)expression at 24h after cerebral ischemia by immunohistochemistry,western blot and qRT-PCR.And the neurological deficits,brain water content and infarct volume were explored.MethodsAt 24h after focal cerebral ischemia,NF-κB expression increased and PPARγdecreased.NF-κB expression was dramatically decreased and PPARγwas increased by telmisartan adminstration,and the neurological deficits,brain water content and infarct volume were alleviated,which were all reversed by GW9662(PPARγspecial antagnist)co-administration.ConclusionPPARγis the up-stream regulator controlling NF-κB expression.Telmisartan inhibited NF-κB expression by PPARγpathway in protecting brain against ischemia.
brain ischemia;NF-κB;peroxisome proliferator-activated receptors
R743.33
A
1007-3205(2013)05-0497-04
2013-03-19;
2013-04-15
祝春華(1978-),女,河北唐山人,河北醫(yī)科大學(xué)第二醫(yī)院主治醫(yī)師,醫(yī)學(xué)博士,從事腦血管病診治研究。
10.3969/j.issn.1007-3205.2013.05.001

