Simultaneous Determination of Inositols and Carbohydrates in Different Citrus Juices by Gas Chromatography with Pre-column Derivatization
ZHANG Yao-hai,ZHAO Qi-yang,ZHANG Xue-lian,WANG Lei,JIAO Bi-ning,,*,ZHOU Zhi-qin
(1. Institute of Citrus Research, Southwest University, Chongqing 400712, China;2. College of Horticulture and Landscape Architecture, Southwest University, Chongqing 400716, China;3. College of Food Science, Southwest University, Chongqing 400716, China)
Simultaneous Determination of Inositols and Carbohydrates in Different Citrus Juices by Gas Chromatography with Pre-column Derivatization
ZHANG Yao-hai1,2,ZHAO Qi-yang1,ZHANG Xue-lian3,WANG Lei1,JIAO Bi-ning1,3,*,ZHOU Zhi-qin2
(1. Institute of Citrus Research, Southwest University, Chongqing 400712, China;2. College of Horticulture and Landscape Architecture, Southwest University, Chongqing 400716, China;3. College of Food Science, Southwest University, Chongqing 400716, China)
A gas chromatographic method using pre-column derivatization was described for the quantitative analysis of fructose, glucose, sucrose, chiro-, scyllo- and myo-inositol in different citrus juices. Juices from different fresh citrus (looseskin mandarin, sweet orange, pummelo, lemon and kumquat) were prepared in the laboratory. Inositols and carbohydrates were analyzed by GC as their oximes derivatives and their identities were confirmed by retention of pure standards. The method was evaluated for precision and recovery using methyl-α-D-glucoside as an internal standard. The recoveries of the method evaluated at two spiked levels were in the range of 98.1%-106.9% with RSDs from 0.6%-6.1%. The limits of detection (LODs) were from 0.29×10-3-0.41×10-3μg/L (RSN=3). The results support the suitability of the method. The method is simple, quick and reproducible, and applicable to confirm inositols and carbohydrates in different kinds of citrus juice.
gas chromatography (GC);inositol;citrus juice;internal standard
It is well known that fruit juices are an important source of energy in the form of glucose, fructose and sucrose being the most abundant in fruit and fruit products[1]. At present, analytical methods of sugar can be roughly divided into two groups. One is chromatography and the other is enzymology. As dominating method, chromatography developed to determine sugar includes gas chromatography (GC)[1-6], high performance liquid chromatography (HPLC)[7-8], high performance anion-exchange chromatography (HPAEC)[9-10]and capillary electrophoresis (CE)[11].
The biochemical meaterials in citrus such as inositols, flavonoids, limonoides, carotenoids and phenolic acids not only have effects of reducing blood sugar level, cholesterollowering, anti-cancer, prevention and treatment of circulatory and psychiatric disorders and so on, but also can be used as the markers of screening species initially and adulteration detection of orange juice[12-13]. Untill now, a few researches on myo-inositol in citrus have been reported, while rarely on chiroand scyllo-inositol. Both the simultaneous determining method of inositols and carbohydrates in citrus juice and the contents in various of citrus are all lace of systematic research. As an important kind of carbohydrates, inositols are present in plants as minor components and some of them have positive physiological effects in humans[14-15]. Myo-inositol is a minor component of fruits[1]. Scyllo-inositol, which has been detected in grapes, has been proposed, along with myo-inositol, to control the genuineness of the concentrated rectified grape[16]. Myoinositol content and myo-inositol/fructose ratio have been found to provide information on the quality and genuineness of citrus juice[2]. The structure of three inositols was shown in Fig.1.

Fig.1 Structure of inositols
Analytical methods developed to determine inositols include titrimetry[17], spectrofluorimetry[18], thin-layer chromatography (TLC)[19], GC[1-6], HPLC[20-21], HPAEC[9-10]and CE[22]. Those traditional analytical methods, such as titrimetry, spectrofluorimetry and TLC, are not suitable for multi-carbohydrates analysis. Although carbohydrates are detected using HPLC and HPAEC without derivatization, HPLC is required to combine with refractive index detector (RID) with poor selectivity and limited sensitivity and also HPAEC is in need of special detector and expensive sugar column. CE is a powerful separation technique that can provide high speed and low cost with poor reproducibility, compared with other chromatographic methods. Since inositol is present in citrus juice in very low concentrations compared to the major carbohydrates, GC technique seemed to be more suitable for its accurate determination. Most of GC separation is carried out using FID (flame ionization detector) due to its response to most volatile organic compound. As inositols and carbohydrates have poor volatility, derivatizing becomes a necessary procedure to determine them.
In the present paper, we have reported a quantitative GC method for the occurrence and contents of inositols in fresh juices from different citrus (loose-skin mandarin, sweet orange, pummelo, lemon and kumquat) in an attempt to establish if these parameters can be used as indicators of the quality and genuineness of citrus juice. Major carbohydrates have also been determined.
1 Materials and Methods
1.1 Meterials, reagents and apparatus
Methanol, hexamethyldisilazane (HMDS) and trifluoroacetic acid (TFA) were analytical reagent (Sinopharm Chemcial Reagent Co. Ltd., Shanghai, China). D-fructose (CAS No. 57-48-7), glucose (CAS No. 50-99-7) and sucrose (CAS No. 57-50-1) were of chromatographic grade. Methylα-D-glucoside, Myo-inositol (CAS No. 87-89-8, ≥99.0%) were obtained from Fluka Company (CAS No. 97-30-3, ≥99.0%, sum of enantimers, Lithuania). D-chiro-inositol (CAS No. 643-12-9), L-chiro-inositol (CAS No. 551-72-4) and scylloinositol (CAS No. 488-59-5) were purchased from Tokyo Chemical Industry Co. Ltd. (EP, Japan).
All other reagents were of analytical grade and deionized water purified by a Milli-Q system (Millipore, Bedford, MA, USA) was used throughout.
The GC analyses were performed on Agilent gas chromatograhy (model 6890, USA) equipped with FID. Three different capillary columns, including HP-1701 (30 m×0.32 mm, 0.25 μm film), HP-1(30 m×0.25 mm, 0.25 μm film) and HP-5MS (30m×0.32mm, 0.25 μm film) were used for the optimization of the experimental conditions.
1.2 Methods
1.2.1 Samples and sampling
Citrus samples were supplied by National Citrus Germplasm Repository officially established in Citrus Research Institute of Chinese Academy of Agricultural Sciences. Citrus juices were crushed after removing skins (and seeds when necessary) and centrifuged at 12000 r/min during 10 min at 5 ℃. 1.00 mL supernatant and 1.00 mL internalstandard solution were mixed in a 25.00 mL volumetric flask and diluted to the mark with methanol/water mixture (70∶30, V/V). Out of the above solution, one portion of 0.40 mL was transferred to a 10 mL colorimetric tube and stored in an oven at 60 ℃ for at least 12 h.
1.2.2 Derivatization procedure
To the above colorimetric tube containing sample and internal standard, 0.75 mL of 1.25% hydroxylaminechloride in pyridine was added. The mixture was kept for 20 min at 50 ℃. After oximation reaction, 0.35 mL hexamethyldisilazane (HMDS) and 0.035 mL trifluoroacetic acid (TFA) were carefully added to the tube parked in ice-bath. Then samples were persilylated at 60 ℃ for 25 min and centrifuged at 12000 r/min for 5 min.
1.2.3 Chromatographic conditions
Gas chromatographic separation was carried out using a HP-1701 fused silica capillary column. All injections were split, the ratio was 9∶1 and the volume was 1 μL. The flow rates of carrier gas (N2, ≥99.999%), fuel gas (H2, ≥99.999%) and combustion-supporting gas (air, ≥99.999%) were 0.8 mL/ min, 40.0 mL/min and 450.0 mL/min respectively. The injector temperature was 250 ℃. FID detector temperature was 300 ℃. The column temperature program was from 200 ℃ (200℃ for 12 min) to 280 ℃ at 25 ℃/min, then 250 ℃ for 5 min.
2 Results and Analyses
2.1 Optimization of derivation reaction
In order to be detected using GC, those compounds with hydroxyl group, such as carbohydrates, are required of derivation treatment. It has been well documented that HMDS, a widely used silylation reagent produced several by-products when it was directly applied to the derivation of reducing sugars[23]. Multiple peaks found for reducing sugars, which corresponded to the various isomeric forms resulted in severe interference. Once reducing sugars react with methylhydroxylamine hydrochloride, the number of isomeric forms can dramatically decrease when using the silylation procedure. So, ketonic group of reducing sugar is in need of protection before silylation reaction and oximation reaction is an effective approach.
2.1.1 Optimization of oximation reaction
It could be seen easily from Fig.2 that neither oximation reaction temperature nor time had remarkable effect on the derivation of three inositols, which resulted from no carbonyl group of their molecular structure. High temperature could accelerate the derivatization reaction of sucrose as well as the hydrolysis of the reagent[24]. Therefore, the experiment aimed at the best temperature to achieve the best derivatization yield. Fig.2(A) indicates that the effect of different temperature on the peak areas. The optimum temperature (50 ℃) was employed.
Usually, the derivatizing is expected to be performed in a short time with satisfying efficiency. In this experiment, the investigation of suitable reaction time was carefully carried out at 50 ℃. As shown in Fig.2 (B), it was demonstrated that the reaction was completed in 20 min. To get reproducible results, the oximation reaction at 50 ℃ for 20 min was performed.
2.1.2 Optimization of silylation reaction

Fig.2 Effect of oximation temperature (oximation time for 20 min) (A) and oximation time (oximation temperature for 50 ℃) (B) on response ratio
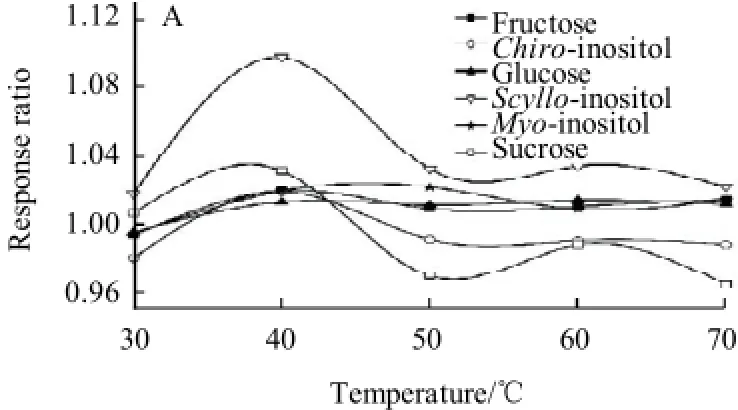
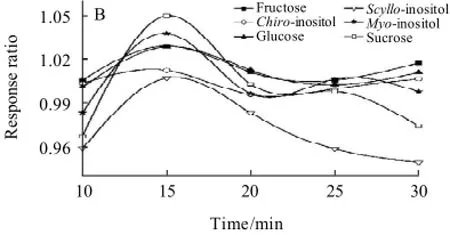
Fig.3 Effect of silylation temperature (silylation time for 25min) (A) and silylation time (silylation temperature for 60 ℃) (B) on response ratio
As shown in Fig.3(A), at first, the peak areas of the derivatives of chiro-inositol, scyllo-inositol and sucrose increased gradually with the increasing of silylation reaction temperature. At 40 ℃, the maximal were almost obtained. The peak areas of their derivatives dropped down markedly with the increase of temperature. In the latter 20 min, the peak areas remained constant. Moreover, the peak areas of other three derivatives could be stable for about 30 min when it reached maximal. Therefore, the silylation reaction was performed at 60 ℃ for 25 min.
Additionally, the influence of how to add silylation reagent on the separation of carbohydrates was studied. When HMDS and TFA were entered to the reaction system at the room temperature, multiple peaks was found with poor separation in Fig.4(A) and Fig.5(A). While at the ice bath, the contrary result was shown in Fig.4(B) and Fig.5(B).
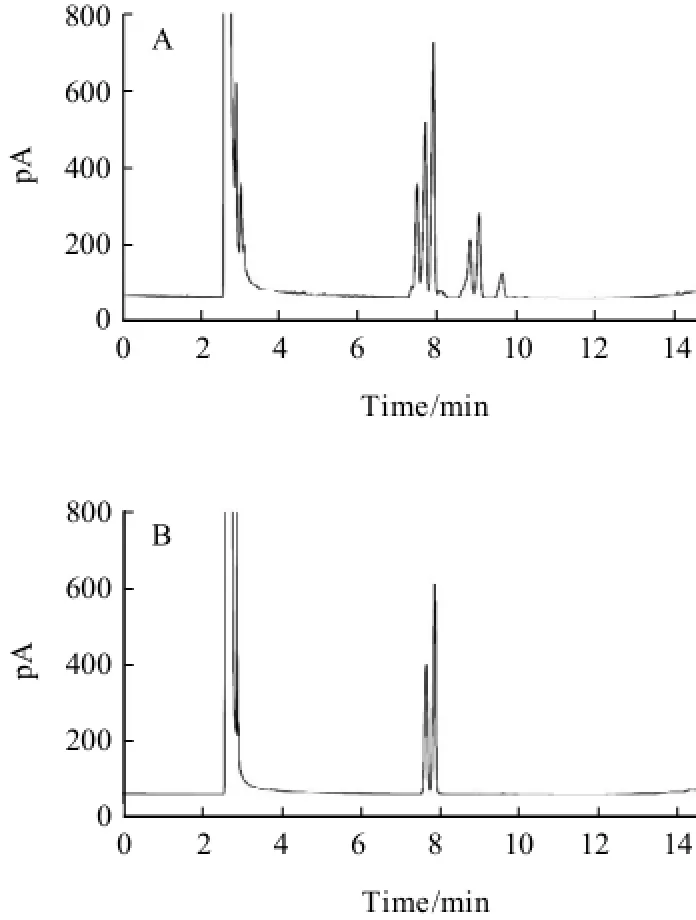
Fig.4 Chromatograms of fructose standard under room temperature (A) and ice-bath (B) conditions
Fig.5 Chromatograms of glucose standard underroom temperature (A) and ice-bath (B) conditions
2.2 Typical chromatogram
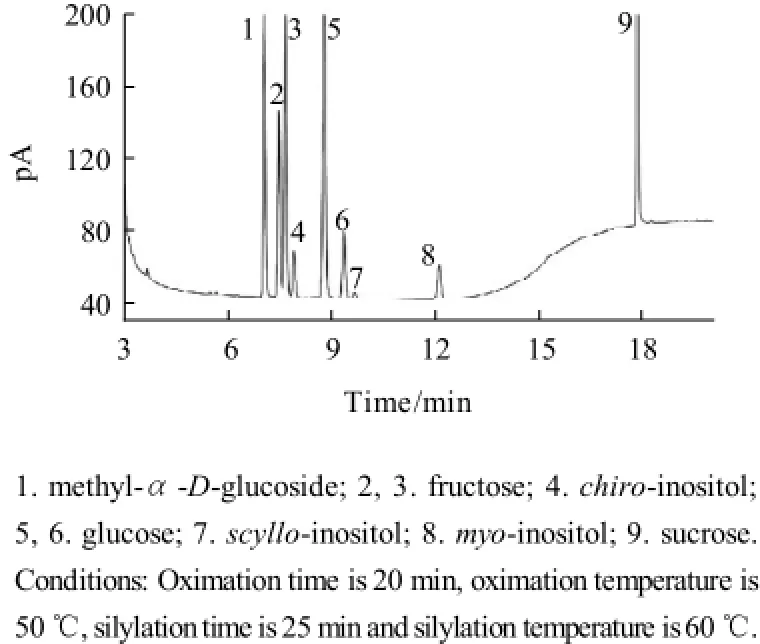
Fig.6 Chromatographic profiles of TMS inositols and sugar oximes of mixed standard resolution
A typical gas chromatogram obtained after use of the optimum conditions for derivatization and separation was shown in Fig.6. The derivatives of inositols and carbohydrates were separated to baseline within 20 min. Although peaks of 2 and 3 from fructose partially overlapped, they were integrated without difficulty. Besides, the effect of different capillary columns on the separation was also taken into consideration. Three types of capillary columns, including HP-1701 (30 m× 0.32 mm, 0.25 μm film), HP-1 (30m×0.25 mm, 0.25 μm film) and HP-5MS (30 m×0.32 mm, 0.25 μm film) were used in the experment. It was observed that chiro-inositol and fruc-tose were not separated to baseline and two peaks of scylloinositol and glucose were completely overlapped either in HP-1 or HP-5MS capillary column. As a result, HP-1701 capillary column was a perfect choice in the separation.
2.3 Accuracy and precision of analytical methods
In order to quantify the recovery, a known amount of each compoud was added to half-diluted freshly squeezed citrus juices. Table 1 gives the analysis results of satsuma mandarin samples. The satisfactory recoveries were found to be 98.1%-106.9% with RSDs ranged from 0.6%-6.1%. The results support the suitability of the method.
2.4 Sample analysis
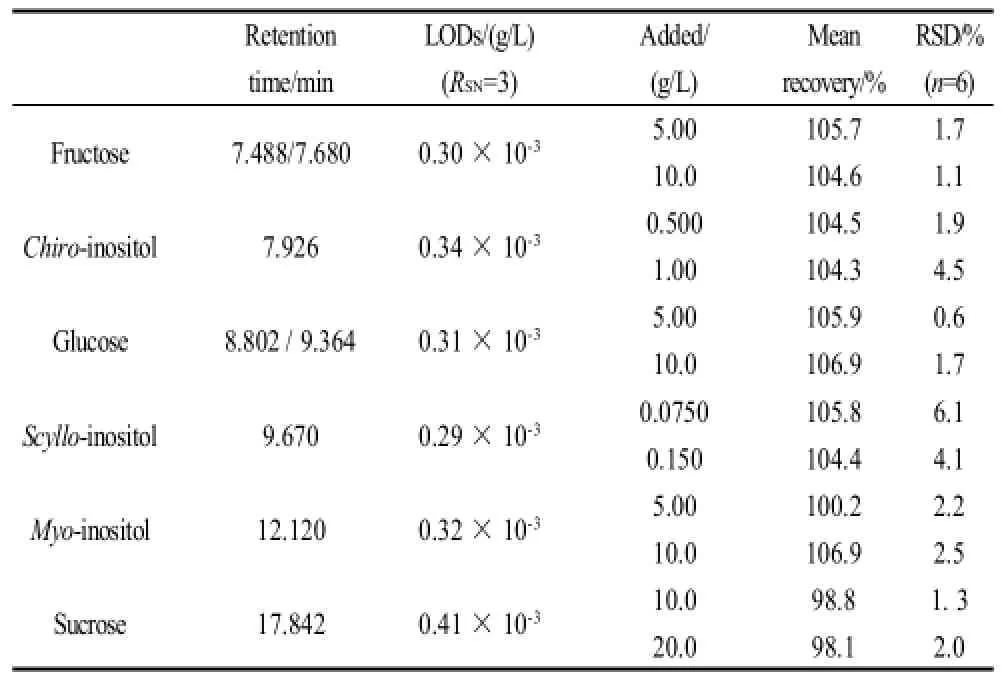
Table 1 Range studied for retention time, limits of quantification (LOQs), relative standard deviation (RSD) and mean recovery of inositols and carbohydrates
The proposed method has been applied to the analysis of citrus samples including loose-skin mandarin, sweet orange, pummelo, lemon and kumquat. Tangerines, mandarins and hybrids were part of loose-skin mandarins. Three major carbohydrates fructose, glucose and sucrose were usually found in a ratio of 1∶1∶2 (m/m). This value was similar to the previous report[6]. Moreover, three inositols have also been found in different fresh citrus juice at low concentrations (Table 2). Chiro-inositol was present in all citrus samples except a portion of pummelos. Scyllo-inositol was found in all citrus juices except a few mandarins, whereas myo-inositol was observed in all analyzed samples.
Myo-inositol was present in variable amounts, from 0.14 g/L in lemon to 3.15 g/L in pummelo. Myo-inositol content in citrus juice except pummelo was within the ranges 0.12-0.16 g/L and 0.13-0.17 g/L reported by Villamiel et al.[2]and Belitz et al.[25], respectively. Myo-inositol in pummelo (0.95-3.15 g/L) was higher than the maximum of above report. Scyllo-inositol ranged from traces in mandarin to 0.43 g/L in hybrid. Chiroinositol varied from traces in pummelo to 1.75 g/L of hybrid.
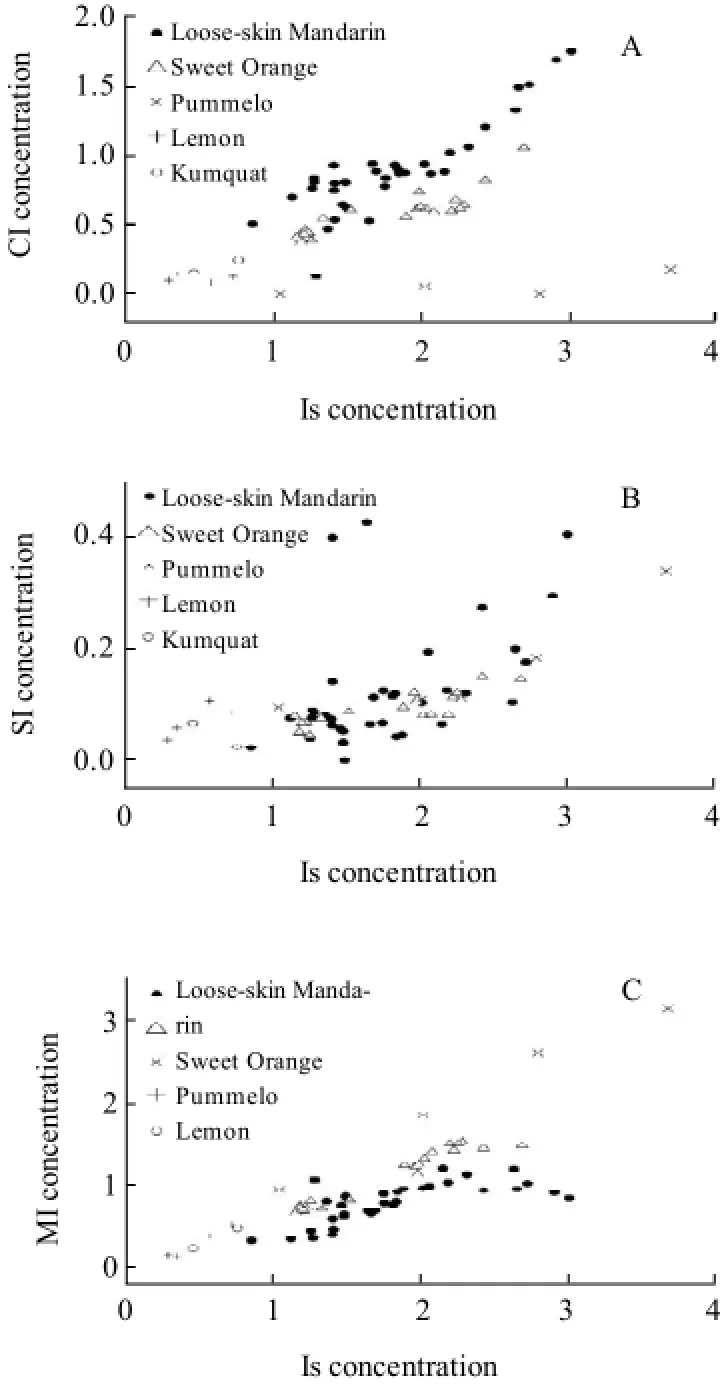
Fig.7 Scatterplot of CI-Is (A), SI-Is (B), MI-Is (C) in citrus juice
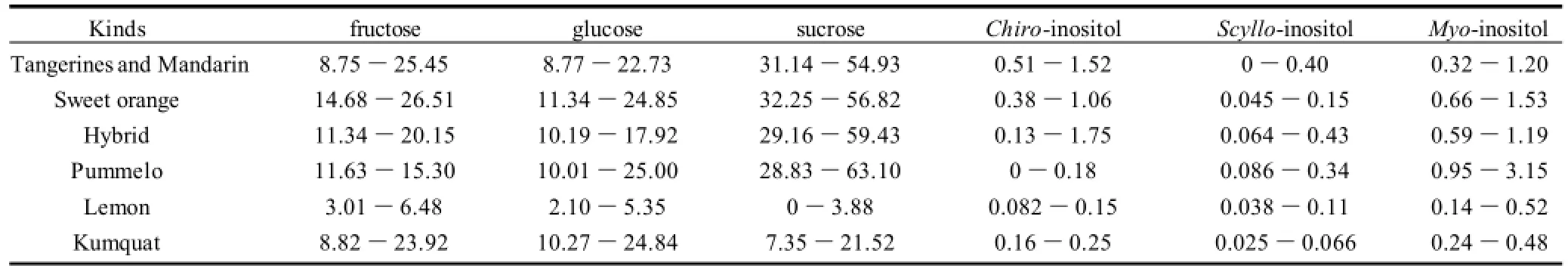
Table 2 Content ranges of fructose, glucose, sucrose, chiro-, scyllo- and myo-inositol in fresh juices from different citrus
Fig.7(A), (B) and (C) show the scatterplots of CI-Is, SI-Is and MI-Is in different citrus juices, respectively. Compared to the scatterplots of MI-Is and CI-Is, the above citrus juices except lemon and kumquat were not distinguished effectively in the scatterplot of SI-Is. On the other hand, it was obvious that different citrus juices were distinguished either in the scatterplots of MI-Is or CI-Is and even the scatterplots of loose-skin mandarin and sweet citrus were seldom or never overlapped. As a result, these parameters such as the scatterplots of MI-Is and CI-Is be used as indicators of the quality and genuineness of citrus juice.
3 Conclusions
From the results above we conclude that our method allows a suitable quantitative determination of inositols and carbohydrates in different citrus juice (loose-skin mandarin, sweet orange, pummelo, lemon and kumquat). Compared with the existing approach, the above is more effective, simple and reproducible. Moreover, the MI-Is and CI-Is scatterplots could afford additional information on the quality and genuineness of commercial citrus juice.
[1]SANZ M L, VILLAMIEL M, MARTINEZ-CASTRO I. Inositols and carbohydrates in different fresh fruit juices[J]. Food Chemistry, 2004, 87 (3)∶ 325-328.
[2]VILLAMIEL M, MARTINEZ-CASTRO I, OLANO A, et al. Quantitative determination of carbohydrates in citrus juice by gas chromatography [J]. Zeitschrift fur Lebensmittel Untersuchung und Forschung A, 1998, 206(1)∶ 48-51.
[3]KATONA Z F, SASS P, MOLNAR-PERL I. Simultaneous determination of sugars, sugar alcohols, acids and amino acids in apricots by gas chromatography-mass spectrometry[J]. Journal of Chromatography A, 1999, 847(1/2)∶ 91-102.
[4]MACIAS-RODRIGUEZ L, QUERO E, LOPEZ M G. Carbohydrate differences in strawberry crowns and fruit (Fragaria×ananassa) during plant development[J]. Journal of Agriculture Food Chemistry, 2002, 50 (11)∶ 3317-3321.
[5]FUZFAI Z, KATONA Z F, KOVACS E, et al. Simultaneous identification and quantification of the sugar, sugar alcohol, and carboxylic acid contents of sour cherry, apple, and ber fruits, as their trimethylsilyl derivatives, by gas chromatography-mass spectrometry[J]. Journal of Agriculture Food Chemistry, 2004, 52(25)∶ 7444-7452.
[6]FUZFAI Z, MOLNAR-PERL I. Gas chromatographic-mass spectrometric fragmentation study of flavonoids as their trimethylsilyl derivatives∶Analysis of flavonoids, sugars, carboxylic and amino acids in model systems and in citrus fruits[J]. Journal of Chromatography A, 2007, 1149(1)∶ 88-101.
[7]MANGAS J J, MORENO J, PICINELLI A, et al. Characterization of cider apple fruits according to their degree of ripening. A chemometric approach [J]. Journal of Agriculture Food Chemistry, 1998, 46(10)∶ 4174-4178.
[8]MASUDA R, KANEKO K, YAMASHITA I. Sugar and cyclitol determination in vegetables by HPLC using postcolumn fluorescent derivatization[J]. Journal of Food Science, 1996, 61(6)∶ 1186-1190.
[9]GUIGNARD C, JOUVE L, BOGEAT-TRIBOULOT M B, et al. Analysis of carbohydrates in plants by high-performance anion-exchange chromatography coupled with electrospray mass spectrometry[J]. Journal of Chromatography A, 2005, 1085(1)∶ 137-142.
[10]BRUGGINK C, MAURER R, HERRMANN H, et al. Analysis of carbohydrates by anion exchange chromatography and mass spectrometry [J]. Journal of Chromatography A, 2005, 1085(1)∶ 104-109.
[11]SOGA T, SERWE M. Determination of carbohydrates in food samples by capillary electrophoresis with indirect UV detection[J]. Food Chemistry, 2000, 69(3)∶ 339-344.
[12]PUPIN A M, DENNIS M J, TOLEDO M C F. Polymethoxylated flavones in brazilian orange juice[J]. Food Chemistry, 1998, 63(4)∶ 513-518.
[13]PETERSON J J, DWYER J T, BEECHER G R, et al. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos∶ a compilation and review of the data from the analytical literature[J]. Journal of Food Composition and Analysis, 2006, 19(Suppl 1)∶ 66-73.
[14]McLAURIN J, GOLOMB R, JUREWICZ A, et al. Inositol stereoisomers stabilize an oligomeric aggregate of Alzehimer amyloid β-peptide and inhibit a β-induced toxicity[J]. Journal of Biological Chemistry, 2000, 275(24)∶ 18495-18502.
[15]NESTLER J E, JAKUBOWICZ D J, REAMER P, et al. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome [J]. The New England Journal of Medicine, 1999, 340(17)∶ 1314-1320.
[16]MONETTI A, VERSINI G, DALPIAZ G, et al. Sugar adulterations control in concentrated rectified grape musts by finite mixture distribution analysis of the Myo- and scyllo-inositol content and the D/H methyl ratio of fermentative ethanol[J]. Journal of Agriculture Food Chemistry, 1996, 44(8)∶ 2194-2201.
[17]ZHANG Yanqiu, LI Zhiwei, ZHANG Baotong, et al. The property of inositol and its application in aquaculture[J]. Feed Industry, 2007, 28 (14)∶ 28-30.
[18]WANG Xiuli, LI Yeyun, ZHOU Hui. Quantitative determination of inositol in herba patriniae[J]. Journal of Anhui Traditional Chinese Medical College, 2002, 21(1)∶ 52-54.
[19]LIU Renjie, PIAO Chunhong, YU Hansong, et al. TLC determination method research of D-chiro-inositol in buckwheat seed[J]. Food Science and Technology, 2006, 8∶ 263-265.
[20]DAI Chuanbo, LI Jianqiao, LI Jianxiu. Determination of inositol by high performance liquid chromatography coupled with refraction detector [J]. Journal of Chemistry Industry and Engineering, 2006, 27(4)∶ 57-58.
[21]YANG Nan, REN G uixing. Determination of D-chiro-Inositol in tartary buckwheat using high performance liquid chromatography with an evaporative light scattering detector[J]. Journal of Agriculture Food Chemistry, 2008, 56(3)∶ 757-760.
[22]KONG Lingyao, WANG Yun, CAO Yuhua. Determination of Myoinositol and D-chiro-inositol in black rice bran by capillary electrophoresis with electrochemical detection[J]. Journal of Food Composition and Analysis, 2008, 21(6)∶ 501-504.
[23]BARTOLOZZI F, BERTAZZA G, DANIELE B, et al. Simultaneous determination of soluble sugars and organic acids as their trimethylsilyl derivatives in apricot fruits by gas-liquid chromatography[J]. Journal of Chromatography A, 1997, 758(1)∶ 99-107.
[24]SHEPHERD T, DOBSON G, VERRALL S R, et al. Potato metabolomics by GC-MS∶ what are the limiting factors[J]. Metabolomics, 2007, 3(4)∶ 475-482.
[25]BELITZ H D, GROSCH W. Quimica de los Alimentos∶ food chemistry [M]. Acribia∶ Zaragoza, 1997.
柱前衍生-氣相色譜法同時測定不同柑橘汁中的糖和肌醇
張耀海1,2,趙其陽1,張雪蓮3,王 磊1,焦必寧1,3,*,周志欽2
(1.西南大學柑桔研究所,重慶 400712;2.西南大學園藝園林學院,重慶 400716;3.西南大學食品科學學院,重慶 400716)
為建立柱前衍生-氣相色譜技術同時測定不同柑橘汁(寬皮柑橘、甜橙、柚子、檸檬和金柑)中3種肌醇(肌肌醇、鯊肌醇、手性肌醇)和3種可溶性糖(果糖、葡萄糖、蔗糖)的方法,以內標物(甲基-α-D-葡萄糖苷)定量,標準物質的保留時間定性。結果表明:在兩個添加水平下,6種成分的平均回收率為98.1%~106.9%,相對標準偏差為0.6%~6.1%,檢測限在0.29×10-3~0.41×10-3μg/L(RSN=3)。本方法簡便快速,結果準確可靠。
氣相色譜;肌醇;柑橘汁;內標物
TS255.1
A
1002-6630(2012)10-0173-06
2011-07-07
國家現代農業(柑桔)產業技術體系建設專項(CARS-27);重慶市自然科學基金項目(CSTC 2009BB1136);
張耀海(1977—),男,助理研究員,博士,研究方向為農產品質量安全檢測。E-mail:zyh26824@sina.com
第四十七批中國博士后科學基金面上項目(20100470808);中央高校基本科研業務費項目(XDJK2012C059)
*通信作者:焦必寧(1964—),男,研究員,研究方向為農產品質量安全檢測。E-mail:bljiao@tom.com

