Localization of stationary pronuclei during conjugation of Paramecium as indicated by immunofluorescence staining
GAO Xin, ZHU Jia-Jun, YANG Xian-Yu, YUAN Jin-Qiang, WANG Yi-Wen, SONG Min-Guo
(The Nurturing Station for the State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University, Lin’an 311300, China)
Localization of stationary pronuclei during conjugation ofParameciumas indicated by immunofluorescence staining
GAO Xin, ZHU Jia-Jun, YANG Xian-Yu*, YUAN Jin-Qiang, WANG Yi-Wen, SONG Min-Guo
(The Nurturing Station for the State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University, Lin’an 311300, China)
After the third prezygotic division during conjugation ofParamecium caudatum, migratory and stationary pronuclei are produced. The migratory pronuclei remain in the paroral region tightly against the conjugating boundaries; while the stationary pronuclei are located beside the migratory pronuclei. To date, however, it is not clear what causes this close side-by-side localization between migratory and stationary pronuclei. In the current study, immunofluorescence staining with monoclonal antibody of anti-α tubulin indicated that “U” or “V” shaped spindles connected the migratory and stationary pronuclei during the third prezygotic division. This observation accounts for the close localization between these two types of pronuclei.
Paramecium; Conjugation; Connecting spindles; Pronucleus
Parameciumcaudatumis a worldwide ciliate species with conjugation, one kind of sexual reproduction. Like other ciliates,P.caudatumhas a polygenomic somatic macronucleus and a diploid germinal micronucleus, both of which are derived from synkaryon (fertilized nucleus) division products (Prescott, 1994; Wichterman, 1986). During conjugation, there are three micronuclear prezygotic divisions before synkaryon formation. The first two are meiotic, whereby the micronucleus divides twice successively to form four haploid nuclei. The third is mitotic, whereby one meiotic product divides once yielding a migratory pronucleus and a stationary pronucleus. The migratory pronuclei remain in the paroral region tightly against the conjugating boundaries. So far, however, there have been few detailed descriptions in regards to the stationary pronuclei, although previous studies have determined that stationary pronuclei are located near the migratorypronuclei (Nanney, 1980; Wichterman, 1986; Nakajima et al, 2001; Santos et al, 2000; Watanabe et al, 1996; Yang & Shi, 2007). This close localization between the two types of pronuclei should benefit the transferred migratory pronuclei to recognize and fuse with the stationary pronuclei to form synkarya. However, it is unknown what makes this close localization between two pronuclei, which is addressed in the current study.
1 Material and Methods
1.1 Chemicals and stock solutions
Monoclonal antibody of mouse anti-α tubulin, FITC-conjugated goat anti-mouse Ig G, propidium iodide (PI), 4% paraformaldehyde, 0.5 mol/L EGTA (pH 8.0), Triton-X 100, and RNase A were purchased from the Beyotime Institute of Biotechnology (Haimen Jiangsu, China). Other chemicals were obtained from the Hangzhou Dafang Chemical Reagent Inc (China).
1.2 Cell culture and induction of conjugation
Two complementary mating types ofP.caudatumwere collected from East Lake Campus of Zhejiang A & F University (China). Cell culture and conjugation induction followed previous descriptions (Hiwatashi, 1968). Conjugating pairs were isolated by iron-dextran particles (Yang & Takahashi, 1999).
1.3 Immunofluorescence staining
The protocol of immunofluorescence staining with monoclonal antibody of anti-α tubulin is as follows: 1) Cells were fixed in 2% paraformaldehyde diluted in 2 mmol/L phosphate buffer (pH 7.0) containing 25 mmol/L KCl (PBS). 2) Fixed cells were rinsed three times with washing buffer (PBS containing 5 mmol/L MgSO4, 2 mmol/L EGTA, and 0.05% Triton-X 100). 3) Cells were blocked with 1% BSA dissolved in 5 mmol/L NH4Cl. 4). Cells were incubated overnight in 1 000× diluted monoclonal antibody of anti-α tubulin containing 10 μg/mL RNase A. 5) Cells were incubated for 2 h in 500× diluted FITC-conjugated goat anti-mouse Ig G containing 2.5 μg /mL PI after three rinses. The stained cells were observed under a fluorescence microscope. All experiments were performed at room temperature (~25°C) (Yang & Takahashi, 2002).
2 Results
To determine the reason for the close localization between migratory and stationary pronuclei, conjugating pairs before synkaryon formation were immunostained with monoclonal antibody of anti-α tubulin, by which micronuclei and spindles were stained but macronuclei were not (Ishida et al, 1999). The spindles and nuclei on the telophase of three prezygotic divisions were compared.
2.1 The First and the second prezygotic divisions
At the first prezygotic division, the anti-α tubulin antibody recognized long and slender spindles (arrowheads in Fig. 1A), while the PI staining indicated two meiotic products distributed randomly in the cytoplasm (arrows in Fig. 1B). Merged pictures of Fig. 1A and B showed the co-localization of microtubules and micronuclei in orange (arrows in Fig. 1C). At early telophase of the second prezygotic division, as with the first prezygotic division, both slender spindles and four randomly distributed meiotic products were observed (Fig. 1D-F). At late telophase of the second prezygotic division, slender and long spindles were observed and at least one meiotic product was observed in the paroral region (Fig. 1G-I).
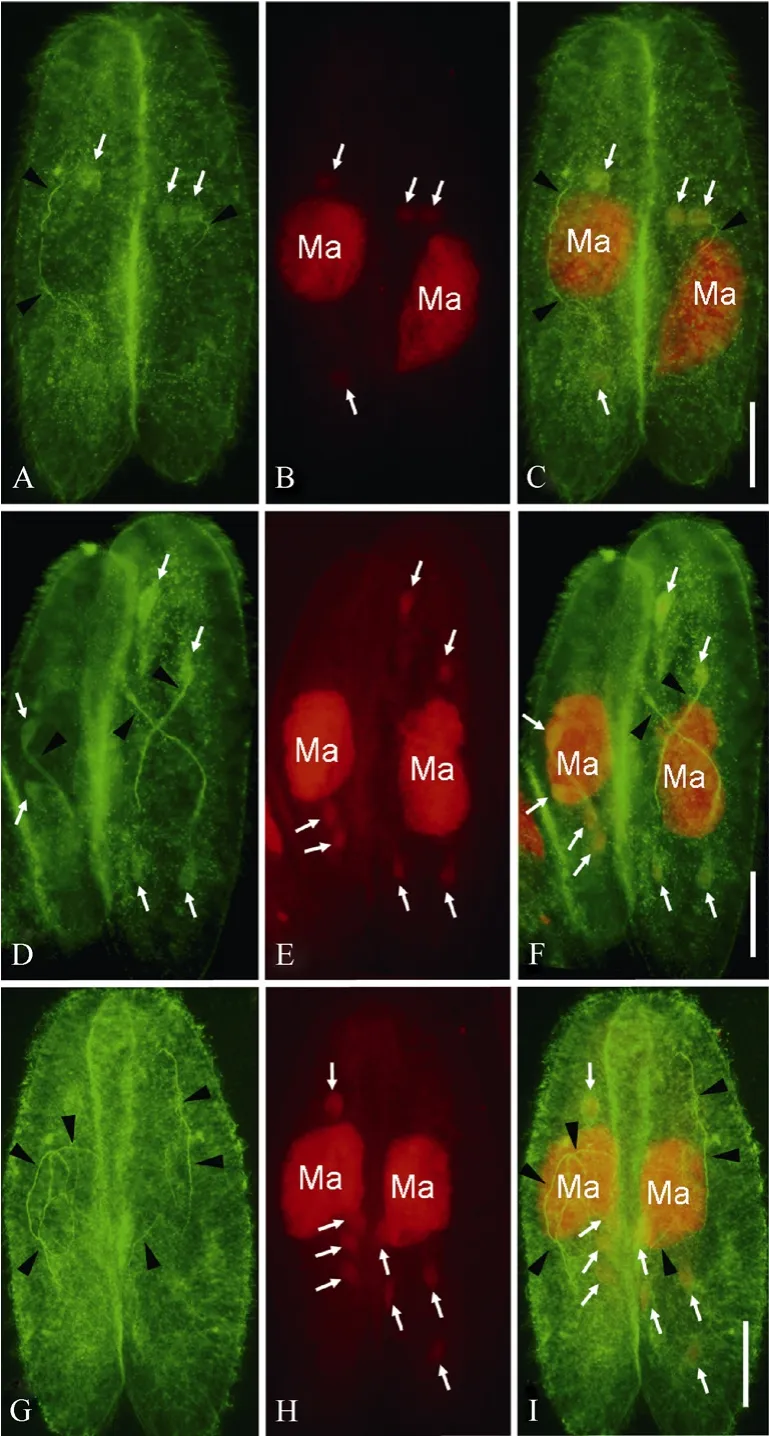
Fig. 1 Meiotic products and spindles during the meiosis of Paramecium caudatum as indicated by double staining of FITC and propidium iodide (PI)
2.2 The third prezygotic division
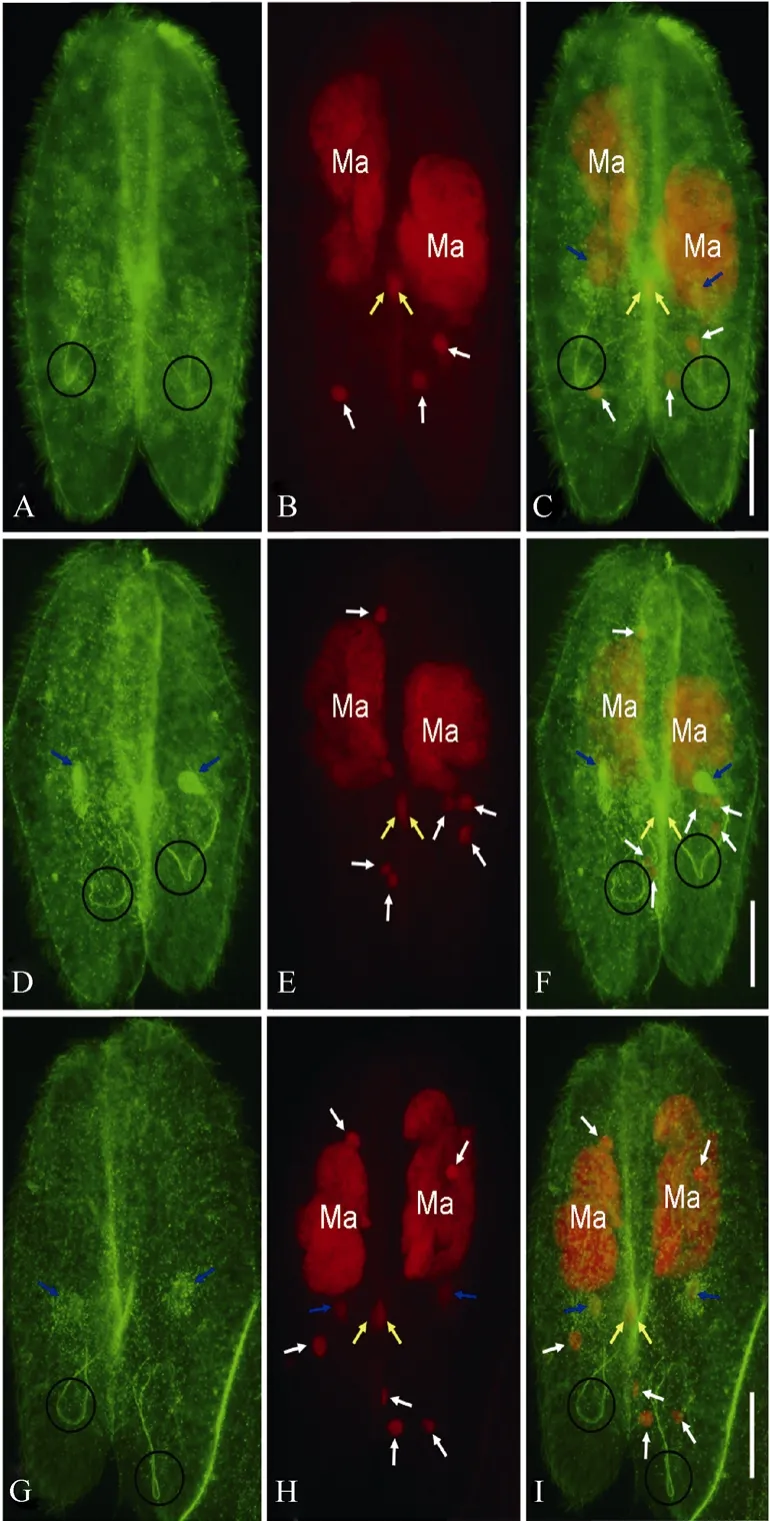
Fig. 2 Prospective migratory pronuclei, stationary pronuclei, and connecting spindles of Paramecium caudatum as indicated by double staining of FITC and PI
After meiosis, only one meiotic product survives in the paroral region, which undergoes mitotic division (the third prezygotic division) to produce migratory “male”and stationary “female” pronuclei, while the other three meiotic products undergo apoptotic degeneration (Gao et al, 2010; Hiwatashi & Mikami, 1989; Yang et al, 2007). The prospective migratory pronuclei (yellow arrows) and stationary pronuclei (blue arrows) were recognized by both the anti-α tubulin antibody and PI (Fig. 2), while the degenerating meiotic products were only recognized by PI (Fig. 2B, E, and H). The slender spindles connecting migratory and stationary pronuclei were recognized by the anti-α tubulin antibody and showed “U”- or “V”-like shapes (Circles in Fig. 2A, D, G). The prospective migratory pronuclei maintained their locations in the paroral region (yellow arrows in Fig. 2). The migratory pronuclei in the two cells of a conjugating pair almost overlapped in the paroral regions, while the prospective stationary pronuclei were located beside the migratory pronuclei (blue arrows in Fig. 2C, F and I). At late telophase of the third prezygotic division, migratory pronuclei and stationary pronuclei were detached from the spindles (Fig. 2G-I), while side-by-side localization between both pronuclei showed no obvious change (compare Fig. 2C, F with I)
3 Discussion
Many studies on conjugation ofP. caudatumhave been conducted since the discovery of the organism (Calkins & Cull, 1907; Hiwatashi & Mikami, 1989; Maupas, 1889). There have also been several studies by immunofluorescence staining with anti-α tubulin antibody (Ishida et al, 1999; Nakajima et al, 2001, 2002; Yang & Takahashi, 2002). Some new details were observed in the current study after conjugating pairs during the three prezygotic divisions ofP. caudatumwere immunostained with anti-α tubulin antibody. 1) Long and slender spindles during meiosis were observed (Fig. 1A, D, G). 2) At late telophase of the second prezygotic division, at least one meiotic product was located in paroral region (Fig. 1H, I). 3) At telophase of the third prezygotic division, the connecting spindles of migratory pronuclei and stationary pronuclei showed“U”- or “V”-like shapes (Fig. 2).
In fact, “U”-shaped spindles connecting migratory and stationary pronuclei during the third prezygotic division have also been observed inP. polycaryum(Yang & Shi, 2007), and “U”- or “V”-shaped spindles have been indicated by protargol inP. caudatum(Gao et al, 2011). If connecting spindles between prospective migratory and stationary pronuclei are stretched straightly, the two pronuclei will be located far from each other. However, “U”- or “V”- shaped spindles allows the two pronuclei to be located side-by-side, which should help transferred migratory pronuclei to easily recognize and fuse with stationary pronuclei to complete synkaryon formation, the most important process during sexual reproduction. Side-by-side localization of the two pronuclei has been observed inTetrahymenaand other species ofParamecium(Snatos et al, 2000; Watanabe et al, 1996). Further studies are needed to determine if this phenomenon is common in ciliates and if these nuclear divisions exist in other organisms.
Calkins GN, Cull SW. 1907. The conjugation ofParameciumaurelia(caudatum) [J].Arch Protistenkd, 10: 375-415.
Gao X, Zhang X, Yang X. 2010. Morphological apoptotic characteristics of the post-meiotic micronuclei inParamecium caudatum[J].Eur J Protistol, 46: 243-250.
Gao X, Shi X, Yang X. 2011. Dynamic behaviour of stationary pronuclei during their positioning inParameciumcaudatum[J].Eur J Protistol, 47: 235-237.
Hiwatashi K. 1968. Determination and inheritance of mating types inParameciumcaudatum[J].Genetics, 58: 373-386.
Hiwatashi K, Mikami K. 1989. Fertilization inParamecium: process of the nuclear reorganization [J].Int Rev Cytol, 114: 1-19.
Ishida M, Nakajima Y, Kurokawa K, Mikami K. 1999. Nuclear behavior and differentiation inParamecium caudatumanalyzed by immunofluorescence with anti-tubulin antibody [J].Zool Sci, 16: 915-926.
Maupas E. 1889. Le rajeunissement karyogamique chez les ciliés [J].Arch Zool Exp Gen, 7: 149-517.
Nakajima Y, Mikami K, Takahashi M. 2001. Role of the cytoplasmic and the intranuclear microtubules on the behavior of pronuclei during the conjugation inParameciumcaudatum[J].Proc Jpn Acad:Ser B, 77: 172-177.
Nakajima Y, Ishida M, Mikami K. 2002. Microtubules mediate germnuclear behavior after meiosis in conjugation ofParameciumcaudatum[J].J Eukaryot Microbiol, 49: 74-81.
Nanney DL. 1980. Experimental Ciliatology [M]. New York: John Wiley and Sons.
Prescott DM. 1994. The DNA of ciliated protozoa [J].Microbiol Rev, 58: 233-267.
Santos ML, Lu E, Wolfe J. 2000. Nuclear death in livingTetrahymena: the case of the haploid nuclei [J].J Eukaryot Microbiol, 47: 493-498.
Watanabe T, Shi X, Liu G, Jin M. 1996. Cytological studies of conjugation and nuclear processes inParameciumduboscquiChatton & Brachon 1933 [J].Eur J Protistol, 32 (Suppl): 175-182. Wichterman R. 1986. The Biology ofParamecium[M]. 2nd Ed. New York: Plenum Press.
Yang X, Takahashi M. 1999. Disturbance of determination of germinal and somatic nuclei by heat shock inParameciumcaudatum[J].J Eukaryot Microbiol, 46: 49-55.
Yang X, Takahashi M. 2002. Nuclei may anchor at specific locations during nuclear determination inParameciumcaudatum[J].EurJ Protistol, 38: 147-153.
Yang X, Shi X. 2007. Gametic nuclear exchange during the conjugation ofParameciumpolycaryum[J].JPN J Protozool, 40: 113-121.
Yang X, Gao X, Shi X. 2007. Detection of haploid nuclear death in livingParamecium caudatum[J].JPN J Protozool, 40:123-130.
免疫熒光染色揭示草履蟲接合生殖過程中靜止原核的空間位置
高 欣, 朱嘉駿, 楊仙玉*, 袁進強, 王逸雯, 宋敏國
(浙江農林大學 亞熱帶森林培育國家重點實驗室培育基地,浙江 臨安311300)
在尾草履蟲的接合生殖過程中, 共有三次配前核分裂。在配前第三次分裂結束后, 兩個接合的細胞內均形成一個遷移原核和一個靜止原核。遷移原核位于口旁錐內,而且緊貼于接合面, 靜止原核則位于遷移原核的外側, 兩者呈左右排列, 距離接近。但是, 目前對導致兩種原核近距離的原因尚不清楚。該文通過α-微管蛋白的單克隆抗體對受精核形成前的接合對進行了免疫熒光染色, 結果發現,配前第三次分裂不同于前兩次分裂, 連接遷移原核和靜止原核的核間連絲伸向細胞的后方, 呈“U”或“V”型, 結果導致兩個原核左右排列, 而不是前后排列, 兩者間的距離縮短。這個結果也闡明了造成兩種原核近距離的原因。
草履蟲; 接合生殖; 核間連絲; 原核
Q959.117; Q954.44
A
0254-5853-(2011)04-0461-04
2011-01-24;接受日期:2011-06-28
高欣,男,講師;研究方向:動物學;E-mail:wildlife909@yahoo.cn
10.3724/SP.J.1141.2011.04461
date: 2011-01-24; Accepted date: 2011-06-28
s:國家自然基金項目(30670397; 31071181);教育部留學回國人員科研啟動基金項目([2007]1108);浙江省教育廳一般項目(Y200804256);浙江農林大學科研發展基金項目(2292000030)
*Corresponding author (通信作者), E-mail: xianyu_yang@hotmail.com
- Zoological Research的其它文章
- 鄭氏比蜢線粒體基因組全序列的測定與分析
- 一種有效區分移植細胞和宿主細胞腦損傷模型的建立
- 懸尾應激對小鼠空間記憶及其反轉學習的損傷效應
- Metabolism and thermoregulation between Mrs Hume’s Pheasant (Syrmaticus humiae) and Elliot’s Pheasant (S. ellioti)
- Notch signaling dependent differentiation of cholangiocyte-like cells from rhesus monkey embryonic stem cells
- Afferent and efferent pathways in the visual system of the freshwater snail Planorbarius corneus

