Synthesis,Crystal Structure and Antibacterial Activity of Magnesium(Ⅱ)Complex with N-Benzenesulphonyl-L-phenylalanine and 1,10-Phenanthroline
TAI Xi-ShiDU Lian-CaiZHAO Zeng-Bing
(1College of Chemistry and Chemical Engineering,Weifang University,Weifang,Shandong 261061,China)
(2College of Bioengineering,Weifang University,Weifang,Shandong 261061,China)
(3Department of Chemistry,Qinghai Normal University,Xining 810008,China)
Synthesis,Crystal Structure and Antibacterial Activity of Magnesium(Ⅱ)Complex with N-Benzenesulphonyl-L-phenylalanine and 1,10-Phenanthroline
TAI Xi-Shi*,1DU Lian-Cai2ZHAO Zeng-Bing3
(1College of Chemistry and Chemical Engineering,Weifang University,Weifang,Shandong261061,China)
(2College of Bioengineering,Weifang University,Weifang,Shandong261061,China)
(3Department of Chemistry,Qinghai Normal University,Xining810008,China)
A new complex bis(N-benzenesulphonyl-L-phenylalanine-O)-bis(water-O)-(1,10-phenanthroline-N,N′)-magnesium(Ⅱ) ([Mg(BPPA)2(phen)(H2O)2]·(phen)·2(H2O)),has been prepared and characterized by elemental analysis,IR spectroscopy and single-crystal X-ray diffraction.The results showed that the compound was triclinic, with P1,a=07.0870(10)nm,b=1.32480(15)nm,c=1.6218(2)nm,α=111.854(2)°,β=101.300(10)°,γ=95.645(10)°, V=1.361 1(3)nm3,Z=1,Mr=1 065.45,Dc=1.300 g·cm-3,T=298(2)K,F(000)=558,μ(Mo Kα)=0.176 cm-1,Flack= 0.07(11),R=0.0484,wR=0.1108,The complex formed one dimensional chain structure through intramolecule and intermolecule hydrogen bonds and π-π stacking.The antibacterial assay of the magnesium(Ⅱ) complex and the ligand of N-benzenesulphony-L-phenylalanine was tested using a modified version of the 2-fold serial dilution method.The complex shows considerable antibacterial activity against escherichia coli,bacillus subtilis and staphylococcus white,and the ligand did not show antibacterial activity.CCDC:744536.
N-benzenesulphonyl-L-phenylalanine;magnesium complex;synthesis;crystal structure;antibactenal activity
Magnesium is an indispensable elementin biology.It is involved in several biochemical processes and is an essential cofactor required for the activation of a variety of enzymes[1].And it may increase the strength of cell membranes,support to cell walls′structures and regulate the function of the cell walls[2-3].So magnesium plays an important role in the whole cell and acts as structures and catalysts[4-5].Moreover,it is the metal centre of the chlorophyll which can be photosynthetic,and is related to the mechanism of some drugs[6].Thus,it is referred to the metal of life.It is significance to study on the structure and characteristic coordination of magnesium carefully for making sure about physiological and biochemical mechanisms of all lives[7].
Amino acid is an important physiological active substance.Besides bactericidal,insecticidal and anticancer biological activity,N-acyl-α-amino acids directly participate in biological processes,and are important to biochemistry and organic synthesis[8-9].In this paper,the new magnesium complex of N-acyl-α-amino acid was synthesized,characterized by elemental analysis,IR spectroscopy and single-crystal X-ray diffraction.The complexformed onedimensionalchain structure through intramolecule and intermolecule hydrogen bonds and π-π stacking.The antibacterial activity of the magnesium(Ⅱ) complex and the ligand of N-benzenesulphony-L-phenylalanine was tested.
1 Experimental
1.1 Materials and instrumentation
The following A.R.grade chemicals were used for the preparation of the studied compound:magnesium chloride, 1,10-phenanthroline, L-phenylalanine, benzenesulfonyl chloride,sodium hydroxide.
The carbon,hydrogen and nitrogen content in the newly synthesized compound was determined on a ElementarVarioEL elementalanalyzer.Infrared spectra (4 000~400 cm-1)was determined with KBr optics on a Nicolet AVATAR 360 FTIR spectrophotometer.The crystal structrue was determined by Bruker Smart Apex CCD Area Detector X-ray single crystal diffraction.
1.2 Synthesis of the ligand
10 mmol(1.651 9 g)of L-phenylalanine and 20 mmol(0.8 g)of sodium hydroxide were dissolved in 100 mL of water at room temperature,and added drop by drop 10 mmol(1.766 2 g)of benzenesulfonyl chloride by stirring at room temperature.The reaction solution was kept running for 4 h,then acidified with the solution of hydrochloric acid (V∶V=1∶1)to pH=2.The white solid precipitation were collected by filtration, washed and dried under vacuum.Yield may reach up to over 65%.Anal.Calcd.for C15H15NO4S(%):C,59.02;H, 4.92;N,4.59.Found(%):C,58.78;H,5.02;N,4.56.
1.3 Synthesis of the complex
1.0 mmol (0.305 g)of N-benzenesulphonyl-L-phenylalanine and 1.0 mmol (0.04 g)of sodium hydroxide were added to the 15 mL of CH3OH/H2O (V∶V=2∶1)solution.After being dissolved,0.5 mmol(0.101 5 g)of magnesium chloride was added to the solution.The mixture was continuously stirred for 3 h at refluxing temperature,then 0.5 mmol(0.09 g)of 1,10-phenanthroline was added to the mixture by stirring 3 h at refluxing temperature.The mixture was cooled at room temperature,and was collected by filtration.By evaporation in air at room temperature,the single crystal suitable for X-ray determination was obtained from methanol solution after ten days.Yield 58%.Anal.Calcd.for C54H52MgN6O12S2(%):C,60.82;H,4.88;N, 7.88.Found(%):C,60.85;H,5.92;N,7.93.
1.4 Antibacterial assay
The ligand and complex were dissolved in sterile water and tested against three reference strains for antibacterial activity,respectively.The antibacterial assay was performed using a modified version of the 2-fold serial dilution method[10],in which the concentration of chemical medicine decreased half as many in a sterile culture medium containing broth as the nutrient, and the strains were incubated 16 h in culture medium at constant temperature 37℃after being activated,and misce bene after being added to the test tubes of chemical medicine,then readings were taken after 24 h of incubation at constant temperature 37℃.All other testconditions were standardized.The resultant turbidities in all tubes were estimated visually,and the lowest drug concentrations were found,which is defined MIC.After 48 h of continuous incubation,the MBC were defined,too.
1.5 Crystal structure determination and refinement
A colourless block single crystal with dimensions of 0.48 mm×0.35 mm×0.23 mm was placed on a glass fiber and mounted on a Bruker Smart Apex CCD area detector.Diffraction data were collected by φ-ω scan mode using a graphite-monochromatic Mo Kα radiation (λ=0.071073 nm)at 298 K.A total of 7 192 reflections were collected in the range 1.40°~25.01°,of which 5786 were unique(Rint=0.0222)and 4280 were observedwith I>2σ(I).The data were corrected for Lp factors. The structure was solved by direct methods and refined on F2by full-matrix least-squares techniques with SHELXL-97[11].All non-hydrogen atoms and hydrogen atoms were refined anisotropically and isotropically, respectively.The final R=0.0484,and wR=0.1108(w= 1/[δ2(Fo2)+ (0.05P)2+0.88P],P=(Fo2+2Fc2)/3,Flack= 0.07(11),S=1.024,(Δ/σ)max=0.000).The largest peak in the final difference Fourier map is 218 e·nm-3and the minimum peak is-274 e·nm-3.Molecular graphics were drawn with the program package SHELXTL-97 crystallographic software package[11].The most relevant crystal data for complex are quoted in Table 1.
CCDC:744536.
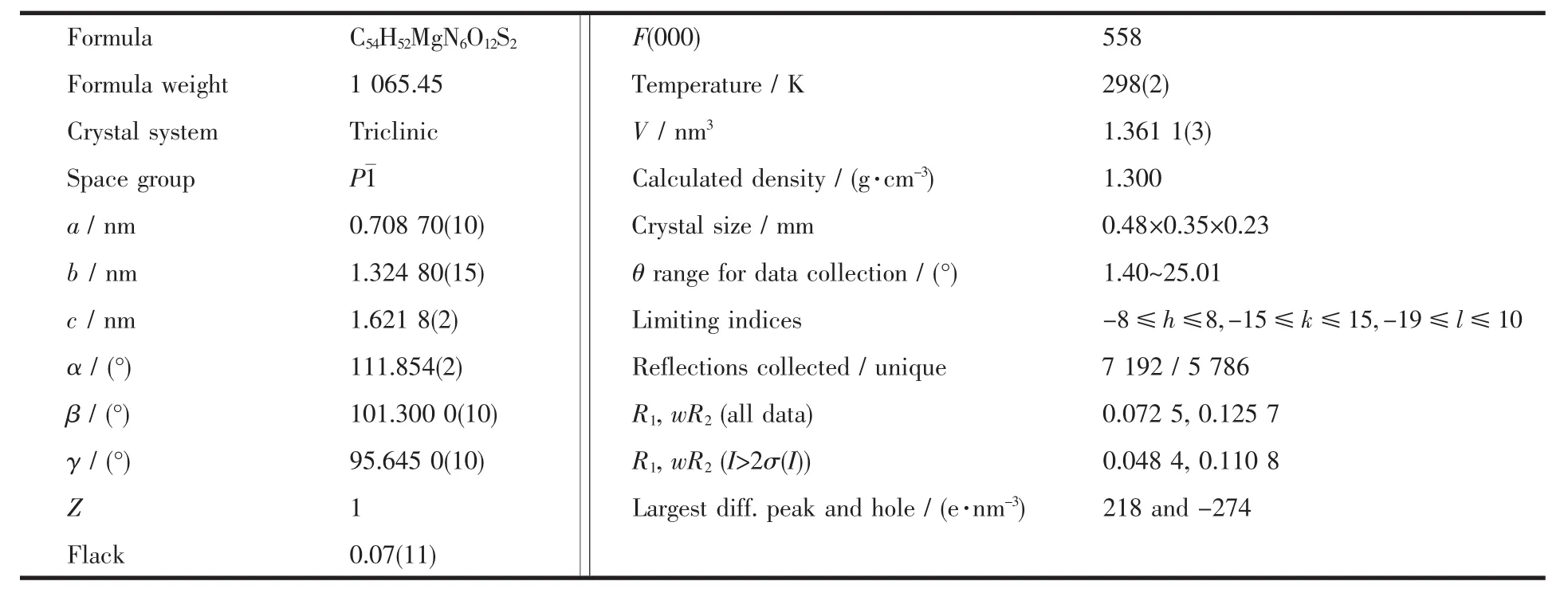
Table 1 Most relevant crystal data for complex
2 Results and discussion
2.1 IR spectra
In the infrared spectra,the ν(COOH)vibrations of the free ligand are at 1 719 and 1 435 cm-1.For the complex,the vibration observed at 1 577 cm-1was assigned as νas(COO-)and that at 1425 cm-1as νs(COO-).It can be explained that the carboxylate oxygen atoms of N-p-tolysulfonyl-L-leucine ligands are involved in the coordination with magnesium atoms,which acted as non-bridging monodentate[12].The bands of the-SO2-NH-groups at 3250~3300,1320 and 1155 cm-1show that there are uncoordinated atoms of the groups, because compared with the free ligand the strong absorption bands are not shifted.In addition,the band at 3 423 cm-1shows that the complex contains water molecules,which are accordance with the results of elemental analyses.
2.2 Crystal structure
Perspective view of the molecule in a unit cell and molecular packing arrangement are shown in Fig.1 and Fig.2,respectively.Selected bond distances and angles are listed in Table 2.
The complex molecule is chiral,which crystallizes in a chiral space group.It can be seen that the coordination environment of the magnesium atom consists oftwo nitrogen atoms from the 1,10-phenathroline,two oxygen atoms from the two N-benzenesulphony-L-phenylalanine groups and two oxygen atoms from the water molecules,making up a distorted octahedral environment.In addition,there is a free 1,10-phenathroline molecule in the complex.The coordination atoms(O(1),O(5),N(3),N(4))are situated equatorial place,and the coordination atoms(O(1w), O(2w))from the waters are situated axial place.The coordination atoms(O(1),O(1w),N(3),O(2w))and (O(5),O(1w),O(2w),N(4))are nearly coplanar,too.The distances of the Mg(1)-N(4)bonds are 0.222 5(4)nm, and the distances of the Mg(1)-N(3)bonds are 0.2258(5) nm.The bond lengths differ only slightly.Mg-O bonds are in the range of 0.202 9~0.208 1 nm,respectively.They are similar to the Mg-O and Mg-N bond lengths reported previously[13-14].Because two nitrogen atoms of 1,10-phenathroline coordinate with the magnesium atom,the five-membered rings have been formed, whose magnesium atoms are coplanar with the ring of 1,10-phenathroline.The benzene rings in the molecule do not show any unusual features,and the bond lengths and bond angles are within the range of normal values.

Fig.1 Molecular structure of the complex,where thethermal ellipsoids were draw at 30%possibility
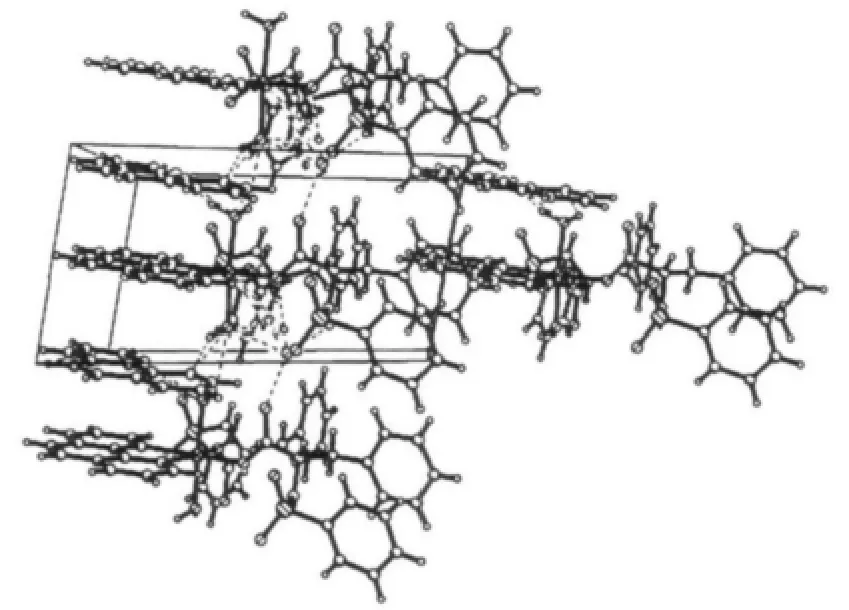
Fig.2 Molecular packing of the complex along the c axis
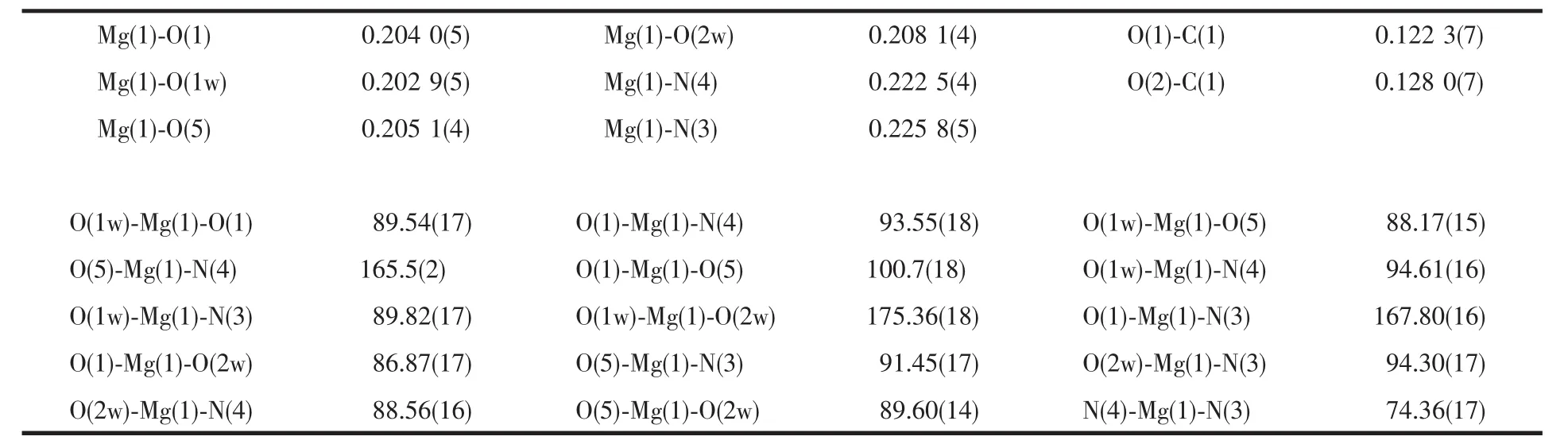
Table 2 Selected bond lengths(nm)and angles(°)of complex
The complex forms one dimensionalchain structrure by hydrogen bonding occurs among intramolecule and intermolecule,which chain parallels the c axis (Fig.3).Between the adjacent 1,10-phenathroline,the dihedral angles are 0.7°and the distance between rings is 0.378 2 nm.So the complexs occur relatively strong π-π stacking,and it is certain that the complex is more stability[15].
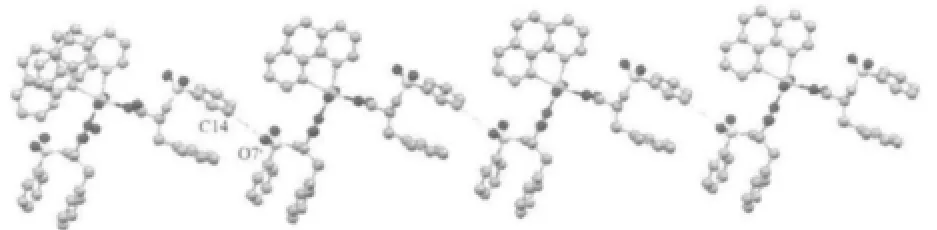
Fig.3 One dimensional chain of the complex
2.3 Antibacterial activity
The antibacterial activity of the N-benzenesulphonyl-L-phenylalanine ligand and Mg(Ⅱ)complex were assayed using three positive(escherichia coli,bacillus subtilis,staphylococcus white)bacterial strains.The antibacterial results of the complex are listed in Table 3.The results indicate that the Mg(Ⅱ) complex shows considerable antibacterial activity,which is better than the results reported previously[15].Compared with the complex, the N-benzenesulphonyl-L-phenylalanine ligand did not show antibacterial activity.So the complex will provide potential applications in the broad spectrum of the antibacterial field.
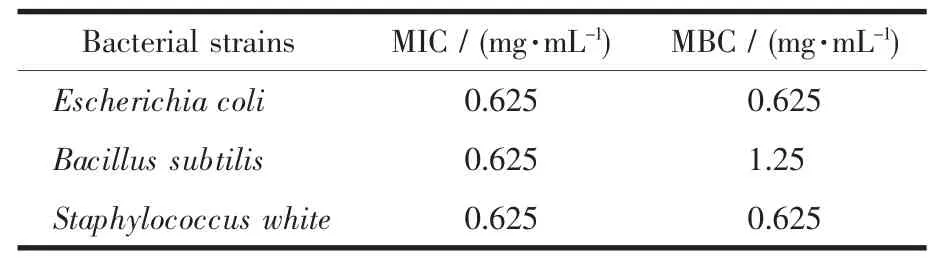
Table 3 MIC and MBC of complex against three bacterial strains
[1]Erxleben A,Schumacher D.Eur.J.Inorg.Chem.,2001,12(10): 3039-3046
[2]SU Yang(蘇揚),ZANG Shuang-Quan(藏雙全),NI Chun-Lin (倪春林),etal.ChineseJ.Inorg.Chem.(WujiHuaxueXuebao), 2004,20(7):845-848
[3]GAO Shan(高山),ZHANG Zhu-Yan(張竹艷),HUO Li-Hua (霍麗華),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2005,21(5):771-774
[4]Miller K B,Caton J S,Finley J W.Biofactors,2006,28(1):33-38
[5]TAI Xi-Shi(臺夕市),XU Jun(許軍),FENG Yi-Min(馮一民), et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25 (3):552-555
[6]Chen Z F,Xiong R G,Zuo J L.et al.J.Chem.Soc.,Dalton Trans.,2000,29(22):4013-4014
[7]YANG Zheng-Yin(楊正銀),YANG Ru-Don(楊汝棟),YU Kai-Bei(郁開北).Acta Chim.Sinica(Huaxue Xuebao),1999,57 (2):236-243
[8]Brown N J,Wu C W,Seurynck-Servoss S L,et al.Biochem., 2008,47(6):1808-1818
[9]Brady S F,Clardy J.J.Am.Chem.Soc.,2000,122(51):12903-12904
[10]ZHOU Guo-Lin(周國林),YAO Quan-Sheng(姚全勝),XU Hui (許暉).Chinese J.Modern Appl.Pharmacy(Zhongguo Xiandai Yingyong Yaoxue),2000,17(2):148-150
[11]Sheldrick G M.SHELXL-97 and SHELXS-97,University of G?ttingen,Germany,1997.
[12]Nakamoto K.Infrared and Ramen Spectra of Inorganic and Coordination Compounds.3 rd Ed.New York:John Wiley and Sons,1978.
[13]Matczak-Jon E,Kurzak B,Kafarski P,et al.Inorg.Biochem., 2006,100(7):1155-1166
[14]Kessler V G,Turevskaya E P,Belokon A I,et al.Polyhedron, 2002,21(16):1629-1634
[15]TAI Xi-Shi(臺夕市),WANG Dong-Fang(王東方),ZHAO Zeng-Bing(趙增兵).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(8):1490-1494
苯磺酰苯丙氨酸、鄰菲咯啉Mg(Ⅱ)配合物的合成、晶體結構及抗菌活性
臺夕市*,1杜連彩2趙增兵3
(1濰坊學院化學化工學院,濰坊 261061)
(2濰坊學院生物工程學院,濰坊 261061)
(3青海師范大學化學系,西寧 810008)
以MgCl2、苯磺酰苯丙氨酸(BPPA)和鄰菲咯啉為原料,合成了一個新型鎂配合物[Mg(BPPA)2(phen)(H2O)2]·(phen)·2(H2O)。對其進行了元素分析、紅外光譜分析,并用X-射線衍射測定了它的單晶結構。結果表明:配合物屬于三斜晶系,P1空間群,配合物分子通過分子內和分子間氫鍵以及π-π堆積作用形成了一維鏈狀結構。初步研究了配體及配合物的抗菌活性,配合物對大腸桿菌、白葡萄球菌及枯草芽孢桿菌有一定的抗菌活性,而配體卻沒有抗菌活性。
苯磺酰苯丙氨酸;Mg(Ⅱ)配合物;合成;晶體結構;抗菌活性
O614.22
A
1001-4861(2011)03-0575-05
2010-09-14。收修改稿日期:2010-11-24。
國家自然科學基金(No.20671073),山東省自然科學基金(No.Y2007B60),山東省中青年科學家獎勵基金(No.2010BSA07004)和濰坊市科技發展計劃資助項目。
*通訊聯系人。E-mail:taixishi@lzu.edu.cn,taixs@wfu.edu.cn
- 無機化學學報的其它文章
- Synthesis,Crystal Structure and Cytotoxicity of Palladium(Ⅱ)Complexes with N-(4-methylbenzoyl)-L-valine Dianion and Aromatic Diimine
- Synthesis,Crystal Structure of Uranium-Potassium Heteronuclear Coordination Polymer
- 鹽湖鹵水萃取提鋰及其機理研究
- 氧化鈦催化羥基磷灰石分解制備可降解磷酸鈣陶瓷
- 菱鎂礦風化石與葉臘石合成堇青石的結構表征
- 微波水熱時間對C/C復合材料結構和抗氧化性能的影響

