雙核Fe配合物[(η5-C5H4)C6H10(C4H3S)Fe(CO)2]2的合成和晶體結構
田利娟馬志宏劉曉煥韓占剛鄭學忠林 進*,
(1河北師范大學化學與材料科學學院,石家莊 050016)
(2河北醫科大學基礎醫學院,石家莊 050017)
雙核Fe配合物[(η5-C5H4)C6H10(C4H3S)Fe(CO)2]2的合成和晶體結構
田利娟1馬志宏2劉曉煥1韓占剛1鄭學忠1林 進*,1
(1河北師范大學化學與材料科學學院,石家莊 050016)
(2河北醫科大學基礎醫學院,石家莊 050017)
由側鏈帶有噻吩的環戊二烯基配體C5H5C6H10C4H3S與Fe(CO)5在二甲苯中加熱回流,合成了1個新穎的四羰基二鐵配合物 [(η5-C5H4)C6H10(C4H3S)Fe(CO)2]2。通過元素分析、IR、1H NMR對其結構進行了表征,用X-射線單晶衍射確定了其結構。X-射線單晶衍射表明配合物中有2個橋羰基和2個端羰基,Fe-Fe的鍵長為0.25465(10)nm。
羰基鐵;結構;環戊二烯基;合成
The coordination chemistry of the cyclopentadienyl have been extensively studied since the ferrocene has been discovered in 1950s[1-2].Replacement of the hydrogen atoms by other substituents alters both the steric and electronic influences of the η5-cyclopentadienyl ring,resulting in differing reactivity and stability of the substituted cyclopentadienyl metal complexes[3].Rencently,people have turned their attention to the heterocyclics of the side chain of cyclopentadienyl[4-6].But few papers have reported on the bearing pendant thienyl of the cyclopentadienyl ligand.Compared to the O-donor and N-donor ligands,the coordination ability of S-donor is sligtly weak.The S atom of the thienyl maybe coordinate to metal center by η1[7-8].So the thienyl side-chain-functionalized cyclopentadienyl ligands can coordinate to the metal center by η5model and by η1model for S atom.The interest of the novel reaction led us to investigate the reactivity of Fe(CO)5with C5H5C6H10C4H3S ligand and determined the structure of the product.
1 Experimental
1.1 General procedures
All procedures were performed under a dry oxygen-free argon atmosphere by using standard Schlenk techniques.A majority of solvents were refined from sodium/diphenyl ketone under an atmosphere of nitrogen.Methylene chloride was distilled over P2O5undernitrogen.Furthermore,the chromatographic spectrum was employed using dichloromethane and petroleum ether as eluent.
IR spectra was recorded as KBr pills with a FTIR 8900 spectrometer.Elemental analysis was performed with a VARIO ELⅢanalyzer and1H NMR spectrum was recorded with a Bruker AV 500 instrument.The ligand C5H5C6H10C4H3S was prepared according to the literature[9].
1.2 Synthesis of the complex
A mixture of the ligand C5H5C6H10C4H3S (0.920 g,4.0 mmol)and Fe(CO)5(0.7 mL,5.0 mmol)of the xylene(35 mL)was stired and refluxed for 14 h.After removal of the xylene,the residue was dissolved and filtered using the methylene chloride.After removal of solvent,the residue was chromatographed on an alumina column using CH2Cl2/petroleum ether(V ∶V=1 ∶2)as eluent.The dark-red band was collected and gave the compound as dark-red crystals(0.78 g,57.2%).m.p.216 ℃.Anal.Calc.for C34H34O4Fe2S2(%):C,59.82;S,9.38;H,5.02.Found(%):C,59.85;S,9.35;H,4.98.IR(ⅤCO,cm-1):1 944s(terminal CO),1 763s(bridging CO).1H NMR(500 MHz,CDCl3,ppm):δ 1.36~1.72(m,20H,(CH2)5),4.09(m,4H,C5H4),5.85(m,4H,C5H4),6.96(d,J=2.0 Hz,2H,C4H3S),7.00(m,2H,C4H3S),7.15(d,J=6.0 Hz,2H,C4H3S).
1.3 Crystal structure determination
The suitable single crystal of compound C34H34O4Fe2S2was prepared in the solvents of hexane/CH2Cl2(V∶V=1 ∶2)which slowly voatilized at room temperature.The crystal data of the title compound was collected at 298(2)K with a Bruker Smart Apex CCD diffractometer,using graphite-monochromated Mo Kα radiation(φ-ω scans, λ=0.071 073 nm).A total of 7 463 reflections were collected and 2643 were unique(Rint=0.0284),of which 2468 were observed(I>2σ(I)).The structure was solved by direct methods with SHELXS-97 program and refined with SHELXL 97 by full-matrix least-squares techniques on F2.All non-hydrogen atoms were refined anisotropically and hydrogen atoms isotropically.Crystallographic data is summarized in Table1.
CCDC:768950.
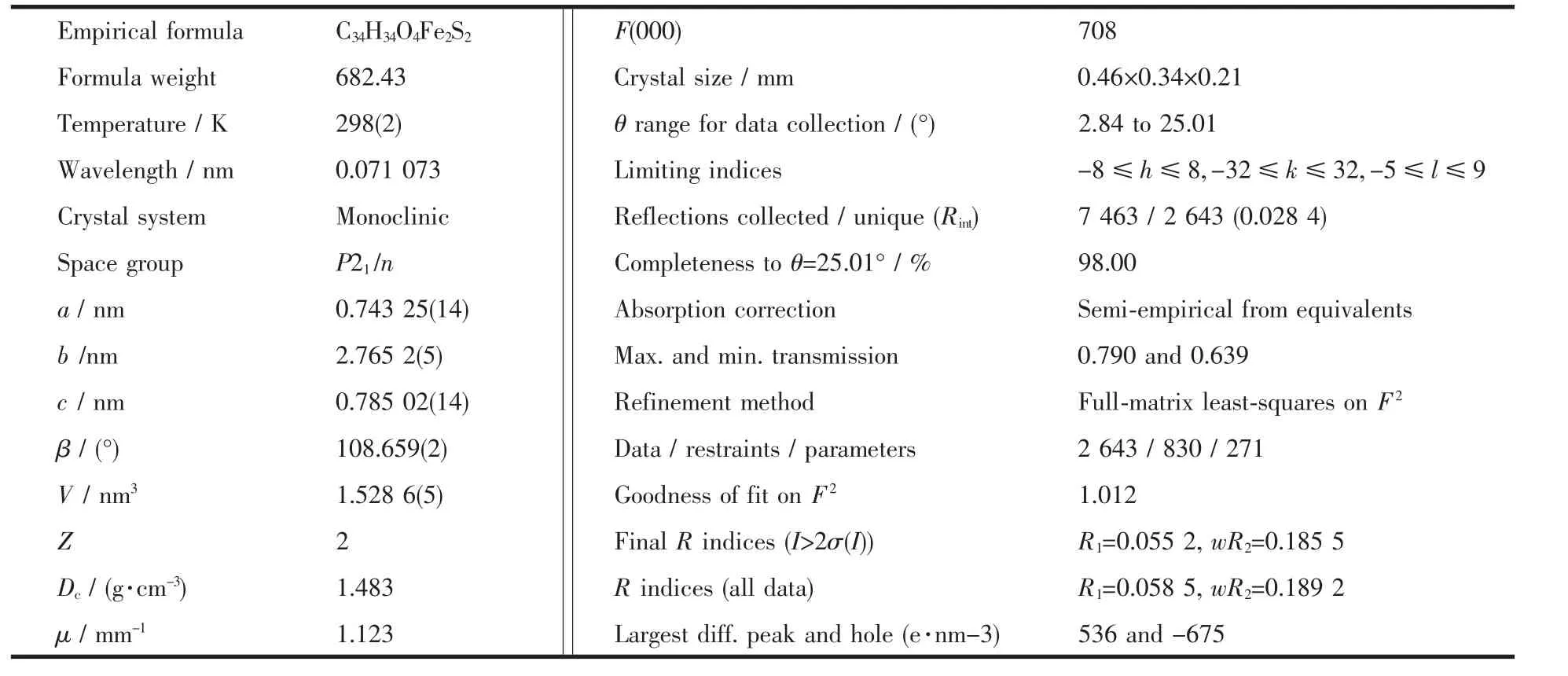
Table 1 Crystal data and structure refinement for the title complex
2 Results and discussion
2.1 Synthesis of complex
When the ligand C5H5C6H10C4H3S reacted with Fe(CO)5in refluxing xylene for 14 h,the corresponding Fe-Fe bonded dinuclear complex[(η5-C5H4)C6H10(C4H3S)Fe(CO)2]2was synthesized.The equation of the reaction is followed(Scheme 1).
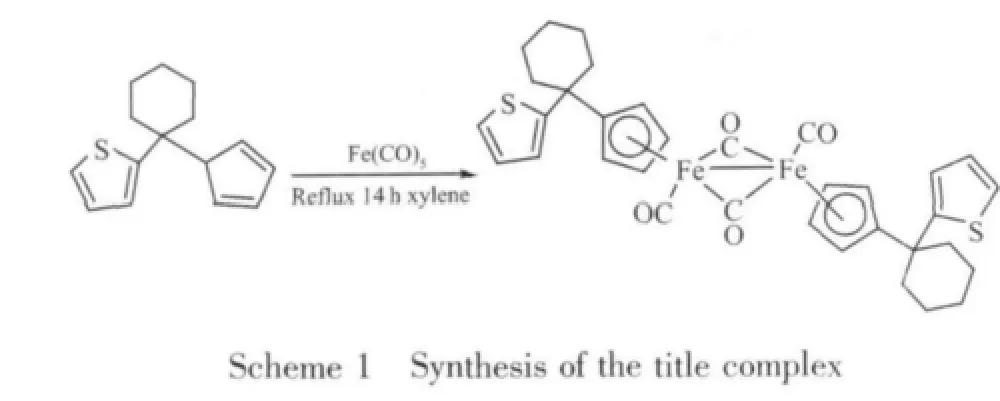
The IR spectrum of diiron complex showed two strong CO absorption peaks at 1944 cm-1in the terminal Ⅴ(CO)region and one at 1763 cm-1in the bridging Ⅴ(CO)region.The1H NMR spectrum showed three groups of peaks for the thienyl protons,two groups for the cyclopentadienyl protons,and a group of multiple peaks for the cyclohexyl protons.The results agreed with the single crystal structure.
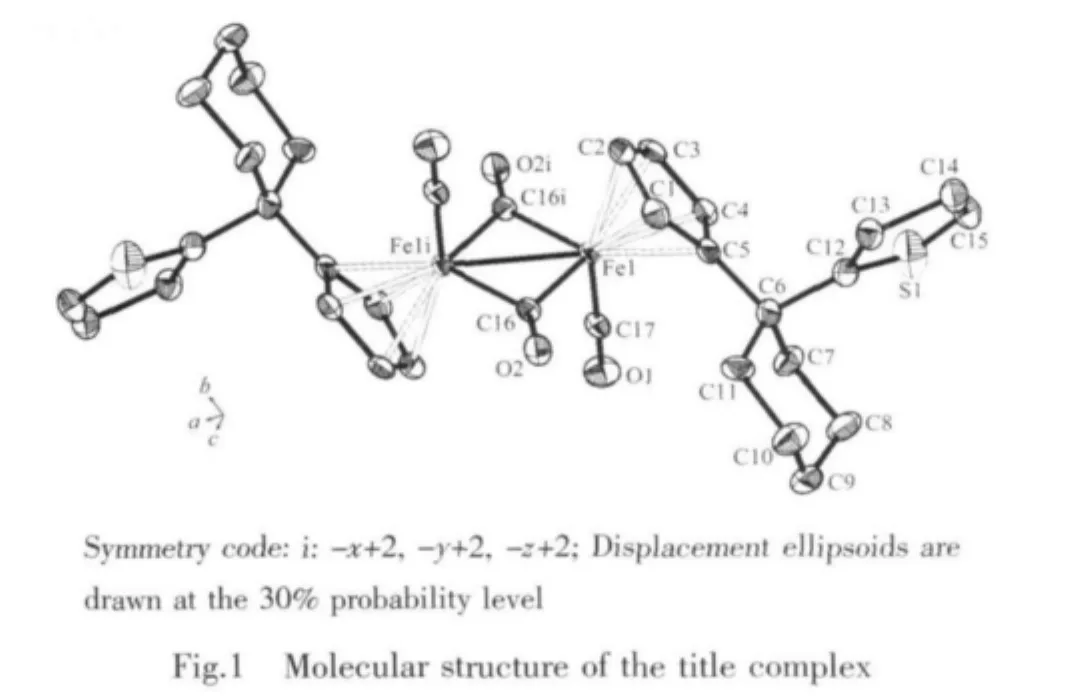
2.2 Crystal structure of the complex
Selected bond lengths and angles are listed in Table 2.The crystal structure of the compound(Fig.1)indicates that it has a trans conformation with two bridging carbonyl ligands and two terminal carbonyl ligands.Besides,the conformation of the cyclohexyl ring is a very steady chair form.And the thienyl rings don′t involve in coordination with the iron atoms.The two cyclopentadienyl rings and the two thienyl rings are parallel,respectively.At the same time,the structure is symmetrical(Ci)and is similar to the trans-[(η5-C5H5)Fe(CO)(μ-CO)]2[11].The bond distance data of the Fe-Fe is 0.25465(10)nm,which is little longer than trans-[(η5-C5H5)Fe(CO)(μ-CO)]2(0.249 0 nm][10]and trans-[(η5-C5Me4H)Fe(CO)(μ-CO)]2(0.253 4(4)nm)[11],and is much shorter than trans-[(η5-C5Me4Ph)Fe(CO)(μ-CO)]2(0.25635(6)nm)[12]and trans-[(η5-C5Me4PhOMe)Fe(CO)(μ-CO)]2(0.25630(8)nm)[12].The bond distance data show thatthe thienylshould have smallsteric hindrance.
Allnon-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms attached to refined atoms were placed in geometrically idealized positions and refined using a riding model,The cyclohexyl and thienyl were disordered over two orientations,with refined site occupation factors of 0.704(6)∶0.296(6),The C-C bond and C-S bond lengths of the disordered cyclohexyl and thienyl were restrained to be the same within a standard deviation of 0.001 nm.The ADPs of C7′C8′C9′C10′and C11′were restrained to be same within a standard deviation of 0.001 nm,the ADPs of C7 C8 C9 C10 and C11 were restrained to be same within a standard deviation of 0.001 nm.The ADPs of C13 C14 C15 and S1 were restrained to be same within a standard deviation of 0.001 nm,the ADPs of C13′C14′C15′and S1′were restrained to be same within a standard deviation of 0.001 nm.
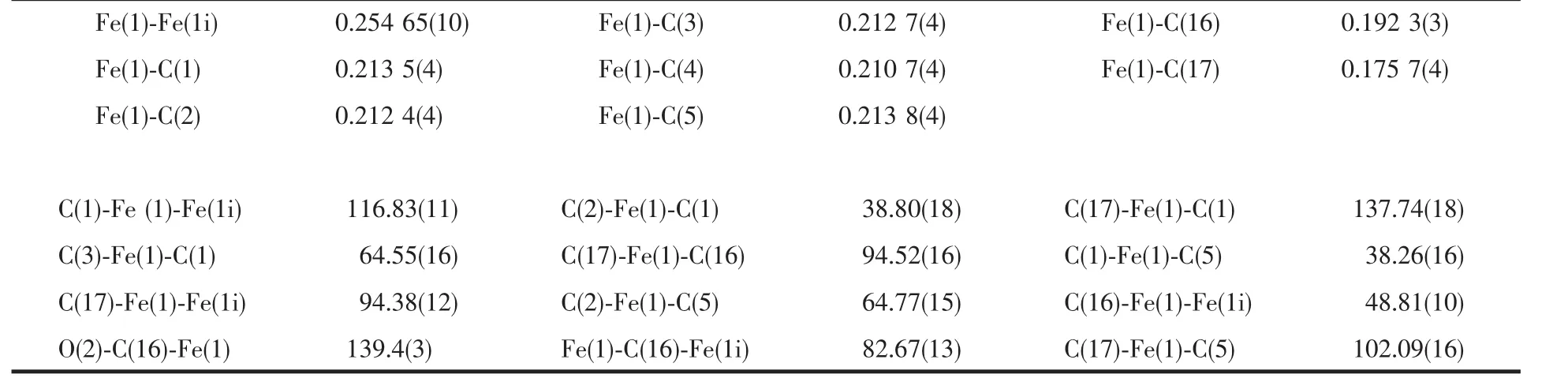
Table 2 Bond distances(nm)and angles(°)for the title complex
In conclusion,a novel compound with the thienyl ring has been synthesized and characterized by the X-ray single crystal diffractometer.The structure issimilar to the[(η5-C5H5)Fe(CO)(μ-CO)]2,which has a trans conformation with two bridging carbonyl ligands and two terminal carbonyl ligands.The difference of the substituents has a little influence on the Fe-Fe bond length.
[1]Kealy T J,Pauson P L.Nature,1951,168:1039-1040
[2]Miller S A,Tebboth J A,Tremaine J F.J.Am.Chem.Soc.,1952,74:2125-2126
[3](a)King R B.Coord.Chem.Rev.,1976,20:155-169(b)Stalke D.Angew.Chem.,Int.Ed.Engl.,1994,33:2168-2171(c)Jutzi P,Burford N.Chem.Rev.,1999,99:969-990(d)Sitzmann H.Coord.Chem.Rev.,2001,214:287-327
[4]Jutzi P,Siemeling U.J.Organomet.Chem.,1995,500:175-185
[5]Siemeling U.Chem.Rev.,2000,100:1495-1526
[6]Butenschn H.Chem.Rev.,2000,100:1527-1564
[7]Balley M F,Dahl L F.Inorg.Chem.,1965,4:1306-1314
[8]Lockemeyer J R,Rauchfuss T B,Rheighold A L,et al.J.Am.Chem.Soc.,1989,111:8828-8834
[9]Xie X M,Huang J L.Appl.Organomet.Chem.,2009,23:1-4
[10]Mills O S.Acta Cryst.,1958,11:620-623
[11]Mcardle P,O′Neill L,Cunningham D.Organometallics,1997,16:1335-1338
[12]Lin J,Gao P,Li B,et al.Inorg.Chim.Acta,2006,359:4503-4510
Synthesis and Crystal Structure of Binuclear Iron Complex
[(η5-C5H4)C6H10(C4H3S)Fe(CO)2]2
TIAN Li-Juan1MA Zhi-Hong2LIU Xiao-Huan1HAN Zhan-Gang1ZHENG Xue-Zhong1LIN Jin*,1
(1College of Chemistry&Material Science,Hebei Normal University,Shijiazhuang 050016,China)
(2College of Basic Medicine,Hebei Medical University,Shijiazhuang 050017,China)
The reaction of the thienyl side chain functionalized cyclopentadienyl ligand C5H5C6H10C4H3S with Fe(CO)5in refluxing xylene gave the novel cyclopentadienyl tetracarbonyl diiron compound[(η5-C5H4)C6H10(C4H3S)Fe(CO)2]2.The structure of the compound was characterized by elemental analysis,IR,1H NMR spectra and X-ray single crystal diffraction.The X-ray crystal structure indicates the structure with bridging and terminal CO groups and the bond length of Fe-Fe is 0.25465(10)nm.CCDC:768950.
carbonyl iron;structure;cyclopentadienyl;synthesis
O614.81+1
A
1001-4861(2011)03-0571-04
2010-05-04。收修改稿日期:2010-11-09。
河北省自然科學基金(No.B2008000150)、河北師范大學博士啟動基金(No.L2005B18)和河北師范大學重點基金(No.L2009Z06)資助項目。
*通訊聯系人。E-mail:linjin64@126.com
- 無機化學學報的其它文章
- Synthesis,Crystal Structure and Cytotoxicity of Palladium(Ⅱ)Complexes with N-(4-methylbenzoyl)-L-valine Dianion and Aromatic Diimine
- Synthesis,Crystal Structure of Uranium-Potassium Heteronuclear Coordination Polymer
- Synthesis,Crystal Structure and Antibacterial Activity of Magnesium(Ⅱ)Complex with N-Benzenesulphonyl-L-phenylalanine and 1,10-Phenanthroline
- 鹽湖鹵水萃取提鋰及其機理研究
- 氧化鈦催化羥基磷灰石分解制備可降解磷酸鈣陶瓷
- 菱鎂礦風化石與葉臘石合成堇青石的結構表征

