新型配合物[Zn(dib)(H2O)3]SO4·2H2O的水熱合成、晶體結構和熒光性質
陳滿生黃妙齡鄧奕芳張春華鄺代治陳志敏
(1功能金屬有機材料湖南省普通高等學校重點實驗室,衡陽師范學院化學與材料科學系,衡陽 421008)
(2泉州師范院化學與生命科學學院,泉州 362000)
新型配合物[Zn(dib)(H2O)3]SO4·2H2O的水熱合成、晶體結構和熒光性質
陳滿生*,1黃妙齡2鄧奕芳1張春華1鄺代治*,1陳志敏1
(1功能金屬有機材料湖南省普通高等學校重點實驗室,衡陽師范學院化學與材料科學系,衡陽 421008)
(2泉州師范院化學與生命科學學院,泉州 362000)
由水熱法合成了鋅化合物[Zn(dib)(H2O)3]SO4·2H2O(1)(dib=1,4-二咪唑基苯),并對其進行了元素分析、IR及X-射線衍射法表征。晶體結構表明:配合物1屬于正交晶系,Fddd空間群。配合物1是由橋聯配體1,4-二咪唑基苯連接成一維鏈狀結構,該一維鏈被氫鍵拓展成三維超分子結構。配合物1的熒光測試研究表明它具有藍色熒光。
鋅配合物;晶體結構;氫鍵;熒光性質
Recently,a great deal of interest in transition metal complex assembly has been devoted to the development of rational synthetic routes to novel one-,two-and three-dimensional crystal frameworks,due totheir potential applications in many areas such as ionexchange,nonlinear optics,molecular sieves,gas storage,catalysis,magnetism,and molecular sensing[1-8].However,the control of formation of supramolecular complexes is still a fascinating challenge at present.Our strategy in this approach is using the organic functionalligand 1,4-di(1-imidazolyl)benzene(dib),which is similar to the 4,4′-bipyridine,only a few structures is known to date[9-12].In order to further investigate the influence of organic carboxylate ligands on the coordiantion architectures and related properties with dib ligands were carried out.Herein we report the synthesis,crystal structure and photoluminescence property of a new zinc coordination polymers,namely[Zn(dib)(H2O)3]·SO4·2H2O(1).
1 Experimental
1.1 Materials and instruments
The regents were used as commercial sources without further purification.Elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer.The ligand dib was prepared as reported previously[13].The IR spectra were recorded on Bruker Vector22 FTIR spectrophotometer using KBr discs.Thermogravimetric analyses were performed on a simultaneous SDT 2960 thermal analyzer under nitrogen with a heating rate of 10 ℃·min-1.The luminescent spectra for the solid powdered samples were recorded at room temperature on an Aminco Bowman Series 2 spectrophotometer with xenon arc lamp as the lightsource.In the measurements of the emission and excitation spectra,the pass width was 4.0 nm.All the measurements were carried out under the same conditions.
1.2 Synthesis of the complex 1
Complex 1 was synthesized by hydrothermal method in a 16 mL Teflon-lined autoclave by heating a mixture containing ZnSO4·7H2O (28.9 mg,0.1 mmol),isophthalic acid(16.7 mg,0.1 mmol),dib(20.1 mg,0.1 mmol)and NaOH (8.0 mg,0.2 mmol)dissolved in 10 mL H2O and heated at 160℃ for 4 d.Block pale yellow single crystals of 1 were collected by filtration and washed by water and ethanol for several times with a yield of 26% (based on dib).Anal.Calcd.for C12H20Zn N4O9(%):C 31.19;H 4.33;N 12.13;found(%):C 31.32;H 4.19;N 12.29.IR(KBr pellet,cm-1):3 419(s),1 524(s),1458(s),1419(w),1373(w),1297(s),1253(s),1113(s),971(w),938(m),824(m),738(m),661(m),618(m).
1.3 X-ray crystallography
The X-ray diffraction measurement for 1 was performed on the Bruker Smart ApexⅡCCD diffractometer with graphite-monochromated Mo Kα radiation(λ=0.071 075 nm)at room temperature.The data were integrated by using the SAINT program[14],which also did the intensity corrections for Lorentz and polarization effect.An empirical absorption correction was applied using the SADABS program[15].The structures were solved by direct methods using the program SHELXS-97 and all the non-hydrogen atoms were refined anisotropically on F2by the full-matrix leastsquares technique using the SHELXL-97 crystallographic software package[16-17].Crystal data and structure refinementparametersarelistedin Table 1.The selected bond lengths and bond angles are given in Table 2.
CCDC:804498.
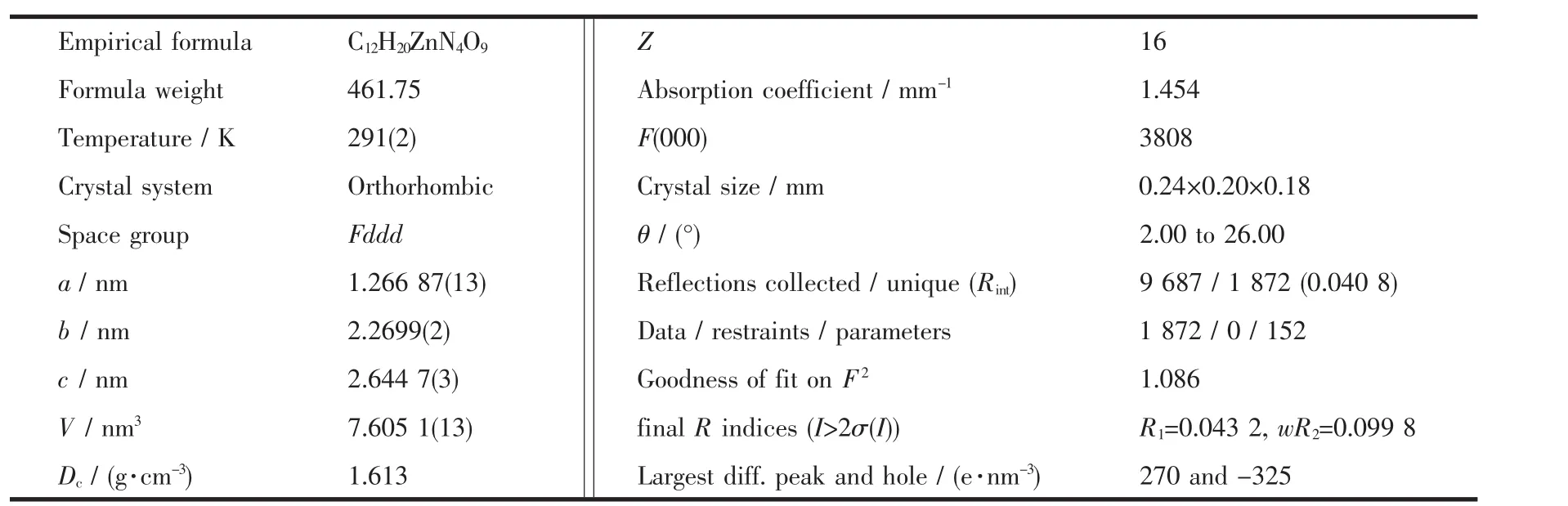
Table 1 Crystal data and structure parameters for complex 1
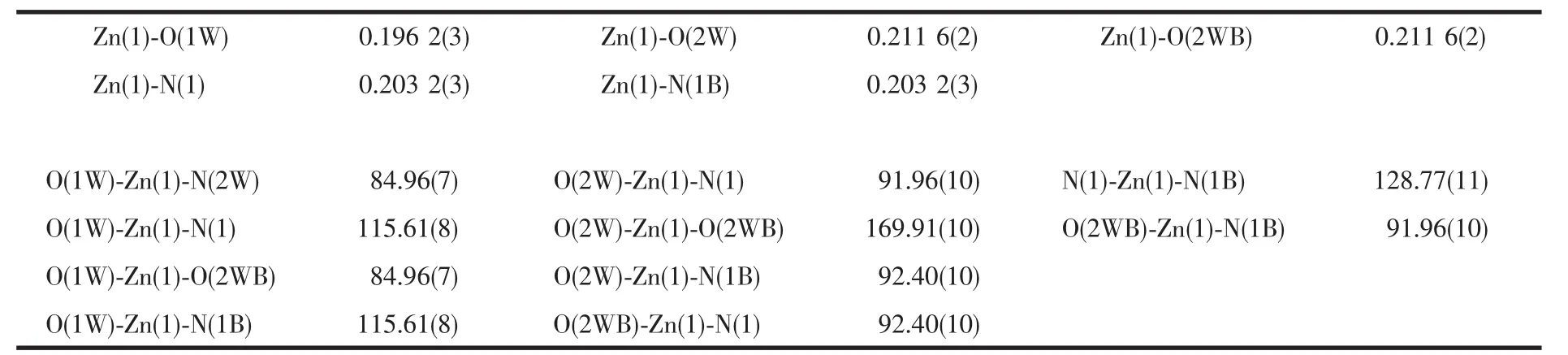
Table 2 Selected bond lengths(nm)and bond angle(°)
2 Results and discussion
2.1 Structure description
The dib reacted with ZnSO4·7H2O under hydrothermal conditions to give complex 1.Singlecrystal X-ray diffraction study reveals an infinite chain of 1 that crystallizes in space group Fddd.The coordination environment around the Zn(Ⅱ)in complex 1 is shown in Fig.1.The central Zn(Ⅱ)atom is located in a distorted trigonal-bipyramidal coordination environment as indicated by an index τ value of 0.69[18]:two N atoms (N1,N1B)from dib ligands and one O atom(O1W)in the equatorial plane and the other two O atoms (O2W and O2WB)from water molecules in axes sites.Three Zn-O bond-distances are 0.196 2(3),0.203 2(3),0.203 2(3)nm,respectively,and two Zn-N bond-distances are the same distance of 0.203 2(3)nm.Then the zinc ions are bridged by dib ligands to form a 1D infinite polymeric chain structure [Zn(dib)]n,alternately.It is noteworthy that coordinated water molecules and the uncoordinated sulfate oxygen atoms provide the hydrogen-bonding donor and acceptor,respectively,resulting in the complex being extended 1D chains into a two-dimensional(2D)supramolecular layer architecture(Fig.2).The 2D layers finally packed together through C-H…O hydrogen bonding interactions to generate 3D supramolecular frameworks.The detailed data of hydrogen bonds for 1 are shown in Table 3.
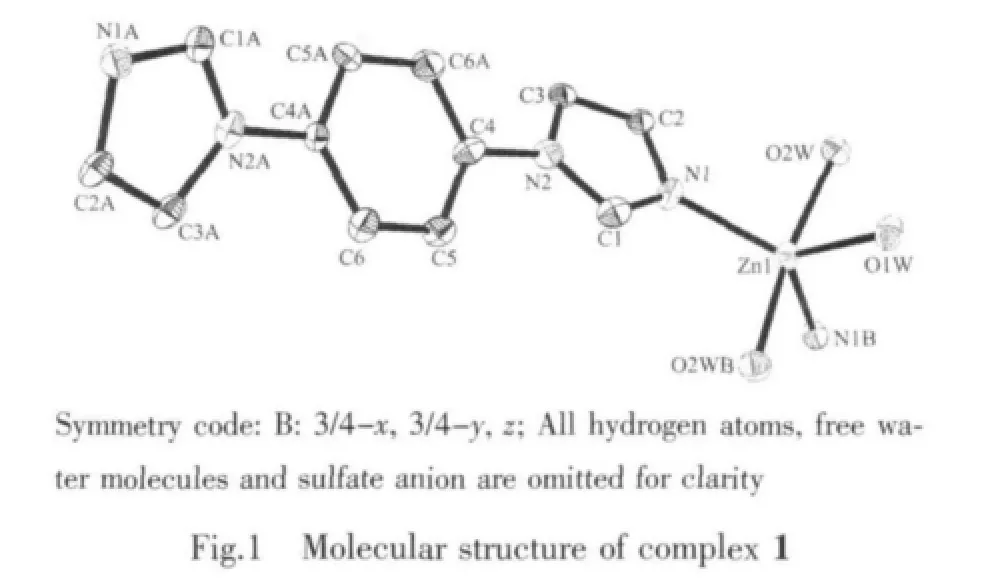
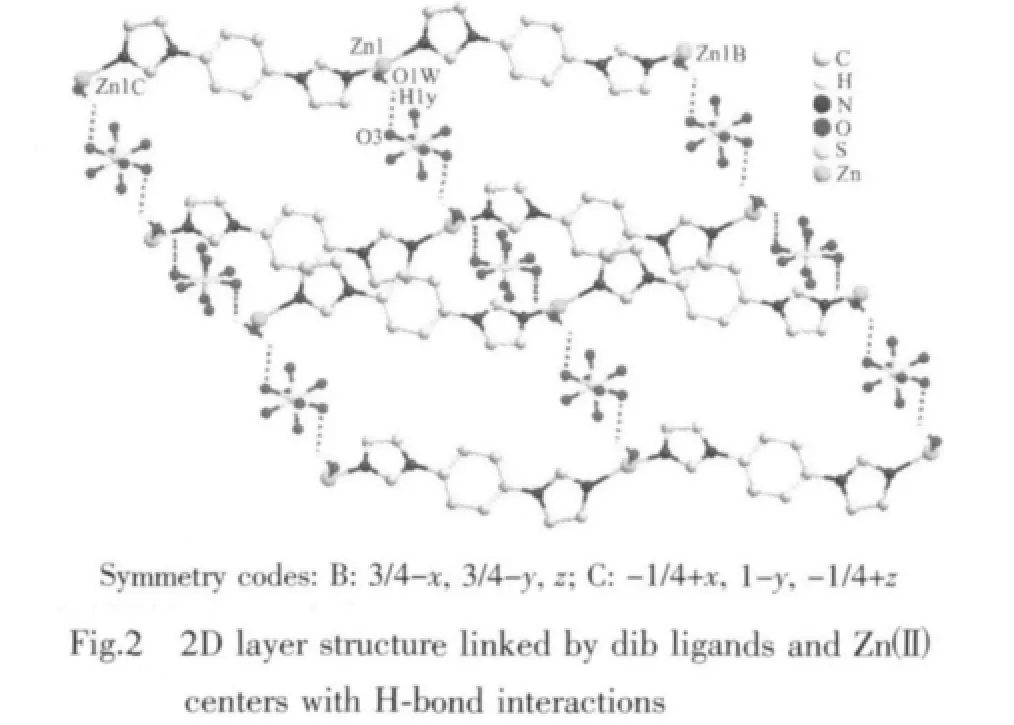
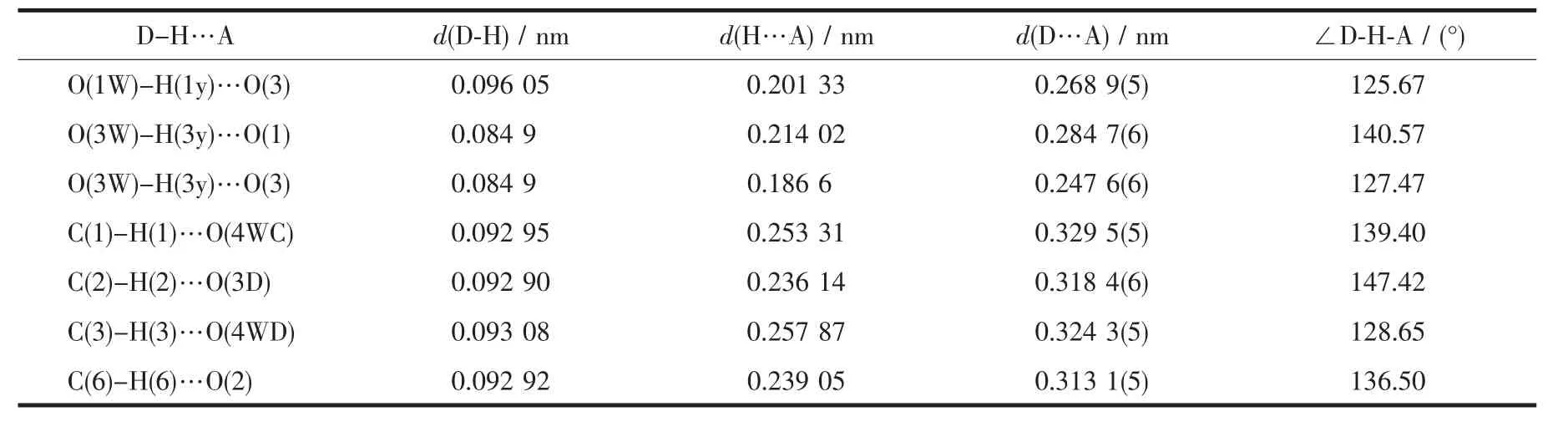
Table 3 Hydrogen bonds for complex 1
2.2 IR,TG and photoluminescence property
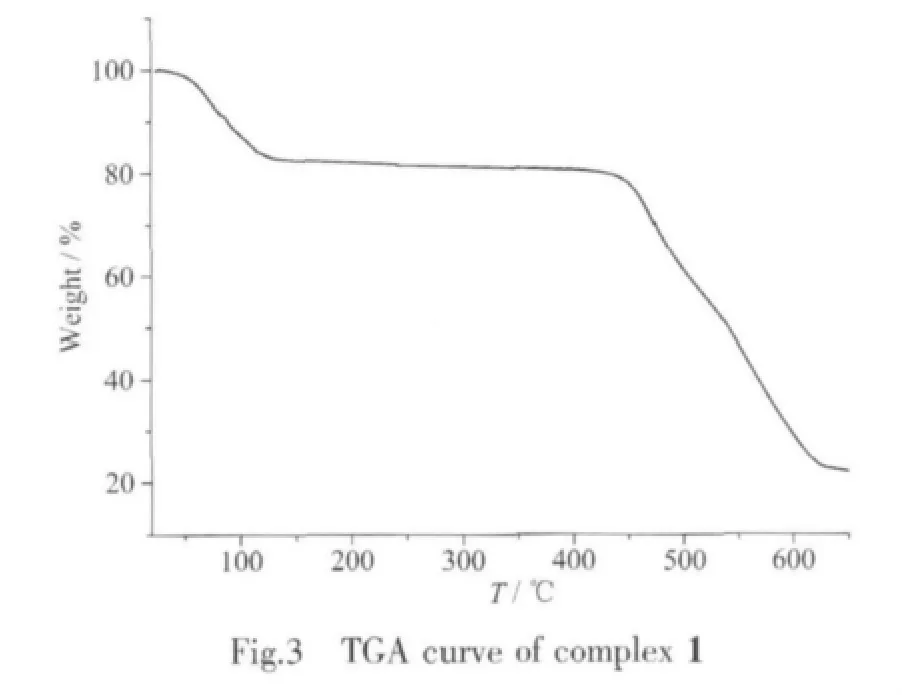
The infrared spectra of the title complex have been recorded and some important assignments are shown above.No strong IR band from-COOH appeared at nearly 1 660 cm-1,indicating that the carboxylate ligands are not existed in it,and peaks at 1113 and 971 cm-1could be assigned to characteristic peaks of SO42-.In the IR spectra,the band at 3 419 cm-1,due to the Ⅴ(O-H)absorptions of water molecules.These IR results are coincident with the crystallographic structural analyses.The results of thermogravimetric analyses(TGA)indicate that complex 1 lost its coordinated and non-coordinated water molecules in the temperature range of 30~155 ℃ (Fig.3).The weight loss of 18.87%is consistent with calculated one of 19.45%.After the loss of all the water molecules,the supramolecular framework is stable up to 400℃,followed by another weight loss at high temperature.
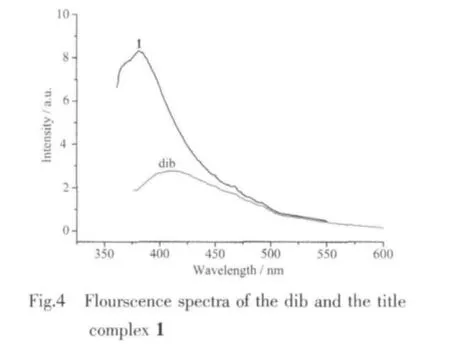
On the otherhand,the photoluminescence property of 1 and free ligand dib was examined in solid state at room temperature.In contrast to the weak luminescence of free dib ligand at ca.408 nm,blue emission was observed for 1 with emission maximum at 383 nm upon excitation at 346 nm(Fig.4).This may be caused by a change in the HOMO and LUMO energy levels ofthe dib ligands coordinating to metalcenters,a charge-transfer between ligands and metal centers,and a corporate contribution of the intraligand transitions or chargetransfer transitions between the coordinated ligands and the metal centers[19],which is different from the reported complex[Cu(dib)1.5(SO4)]·3H2O[9].
[1]Cote A P,Benin A I,Ockwig N W,et al.Science,2005,310:1166-1170
[2]Yaghi O M,O′Keeffe M,Ockwig N W,et al.Nature,2003,423:705-714
[3]Wu C D,Hu A,Zhang L,et al.J.Am.Chem.Soc.,2005,127:8940-8941
[4]Fang Q R,Zhu G S,Xue M,et al.Cryst.Growth Des.,2008,8:319-329
[5]James S L.Chem.Soc.Rev.,2003,32:276-288
[6]Seo J S,Whang D,Lee H,et al.Nature,2000,404:982-986
[7]Eddaoudi M,Kim J,Rosi N,et al.Science,2002,295:469-472
[8]Zhang X M,Hao Z M,Zhang W X,et al.Angew.Chem.Int.Ed.,2007,46:3456-3459
[9]XIE Jing(謝靜),CHEN Xuan(陳軒),LIU Guang-Xiang(劉光祥),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25:1295-1298
[10]Fan J,Hanson B E.Inorg.Chem.,2005,44:6698-7008
[11]Fan J,Yee G T,Wang G B,et al.Inorg.Chem.,2006,45:599-608
[12]Chu Q,Liu G X,Huang Y Q,et al.Dalton,2007:4302-4311
[13]Cristau H,Cellier P P,Spindler J,et al.Chem.Eur.J.,2004,10:5607-5622
[14]SAINT,Version 6.02a,Bruker AXS Inc.,Madison,W1,2002.
[15]Sheldrick G M.SADABS,Program for Bruker Area Detector Absorption Correction,University of G?ttingen,G?ttingen,Germany,1997.
[16]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of G?ttingen,G?ttingen,Germany,1997.
[17]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of G?ttingen,G?ttingen,Germany,1997.
[18]Addison A W,Rao T N,Reedijk J,et al.J.Chem.Soc.,Dalton Trans.,1984:1349-1356
[19]Li S L,Lan Y Q,Ma J F,et al.Cryst.Growth Des.,2008,8:675-684
Hydrothermal Synthesis,Crystal Structure and Luminescence of a New Complex[Zn(dib)(H2O)3]SO4·2H2O
CHEN Man-Sheng*,1HUANG Miao-Ling2DENG Yi-Fang1ZHANG Chun-Hua1KUANG Dai-Zhi*,1CHEN Zhi-Min1
(1Key Laboratory of Functional Organometallic Materials of Hengyang Normal University,Department of Chemistry and Materials Science,Henyang Normaol University,Hengyang,Hunan 421008,China)
(2College of Chemistry and Life Science,Quanzhou Normal University,Quanzhou,Fujian 362000,China)
A new zinc coordination polymers[Zn(dib)(H2O)3]SO4·2H2O(1)was obtained by hydrothermal assembly of ZnSO4·7H2O with a isophthalic acid in the presence of N-donor ligands,namely 1,4-di(1-imidazolyl)benzene(dib).Complex 1 crystallizes in orthorhombic,space group Fddd with a=1.26687(13)nm,b=2.2699(2)nm,c=2.6447(3)nm,V=7.605 1(13)nm3,Z=16,C12H20N4ZnO9S,Mr=461.75,Dc=1.613 g·cm-3,μ=1.454 mm-1,F(000)=3808,Rint=0.0408,R=0.0432,wR=0.0998.Single-crystal X-ray diffraction analysis revealed that each dib ligand in turn uses its two mizale groups to connect two metal centers,then the one-dimensional(1D)chains is formed.On the other hand,the 1D chains are further connected by hydrogen bonds interactions to give a three-dimensional(3D)supramolecular structure.In addition,the luminescent property of complex 1 has been investigated at room temperature,indicating the blue photoluminescence exists in it.CCDC:804498.
Zn(Ⅱ)complex;crystal structure;hydrogen bond;luminescent property
O614.24+1
A
1001-4861(2011)03-0561-04
2010-09-09。收修改稿日期:2010-10-22。
湖南省重點學科建設項目,湖南省青年骨干教師基金(2008),衡陽師范學院青年骨干教師基金(2007),衡陽師范學院科學基金啟動項目(No.10B67)和湖南省教育廳(No.10C0473)資助項目。
*通訊聯系人。E-mail:cmsniu@163.com
- 無機化學學報的其它文章
- Synthesis,Crystal Structure and Cytotoxicity of Palladium(Ⅱ)Complexes with N-(4-methylbenzoyl)-L-valine Dianion and Aromatic Diimine
- Synthesis,Crystal Structure of Uranium-Potassium Heteronuclear Coordination Polymer
- Synthesis,Crystal Structure and Antibacterial Activity of Magnesium(Ⅱ)Complex with N-Benzenesulphonyl-L-phenylalanine and 1,10-Phenanthroline
- 鹽湖鹵水萃取提鋰及其機理研究
- 氧化鈦催化羥基磷灰石分解制備可降解磷酸鈣陶瓷
- 菱鎂礦風化石與葉臘石合成堇青石的結構表征

