新型截短腸毒素C2突變株抑制腫瘤細胞生長
回晶,肖芳,李輝,崔小進,劉宏生,胡風慶
1 遼寧大學生命科學院 生物材料和生物制藥實驗室,沈陽 110036 2 沈陽藥科大學制藥工程學院,沈陽 110016
Introduction
Bacterial superantigens (SAgs) are a class of highly potent immuno-stimulatory molecules mainly produced by Staphylococcus aureus and Streptococcus pyogenes[1]. Staphylococcal enterotoxins (SEs) are typical SAg. Until now, a large number of genetically distinct SAgs are presented in these bacterial pathogens, with more than 40 distinct SAg serotypes[1]. Sequence identities between the various SAgs can range from below 5% for distantly related members to >95% for closely related SAgs. In contrast to conventional antigens, SAgs bind to major histocompatibility complex (MHC) class II molecules on antigen-presenting cells outside the antigen binding cleft and are presented as unprocessed proteins to T-cell receptors (TCR) carrying particular Vβ chains. Very low concentrations of SAgs are able to activate a large amount of resting T cells, thereby releasing massive cytokines, including interleukin (IL)-2, interferon (IFN)-γ and tumor necrosis factor (TNF), which producing significant tumor inhibition in vivo and in vitro[2-3]. Therefore, SE has been extensively employed for the studies of anti-tumor immunotherapy[4-9]. However, the high molecular weight of SEC2 has restricted its clinical applications as agent of anti-tumor immunotherapy due to its poor permeability through the biological barrier[10].
Up to now, although the precise crystal structure of SEC2 has been obtained[11-13], the functions and roles of specific regions and residues of SEC2 have not been completely understood. It was reported that a 6.5 kDa N-terminal fragment of SEC1 possessed a low level of T-cell stimulatory activity and the 22 kDa C-terminal fragment was inactive[14]. A SEA fragment containing residues 107 to 233 had no T-cell stimulatory activity[15]. The fragment containing 180 C-terminal residues retained all activities of the intact SEC1 molecule, and the N-terminus was not required for the superantigen activity of SEC1[16]. The recombinant N-terminal fragment of TSST1 did not stimulate T-cell proliferation whereas the C-terminal domain did[17]. SEC2 mutants lacking 18 or more N-terminal residues severely impaired the superantigen activity[18]. In addition, functional regions responsible for T-cell stimulatory activities did not correlate with those of emetic activity of SEs[19-21]. T cell proliferation efficiency directly correlated with affinity for MHC class II. The disulphide bond and cystine loop are not only an absolute requirement for lymphocyte proliferation but are related to emetic activity of SEs[22-25]. According to these results, it helps to make low molecular weight of derivatives possessing significantly biological effects and lacking side-effects.
In this paper, novel SEC2 mutant (NSM), preserving the important functional sites responsible for the T-cell stimulatory activities but removing the sites responsible for emetic activity according to the results of biological activity assay in vitro, was obtained through truncation of SEC2. NSM efficiently inhibited the growth of tumor cell in vitro. It is valuable for the further research and investigation of in vivo testing so as to provide novel anti-tumor agents with the no side-effects and best treatment effects for clinic.
1 Materials and methods
1.1 Cell lines, bacterial strains, vectors and culture conditions
Human colorectal cancer cells Cx-1 from China Medical University were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) (TBD). Human breast cancer cells MCF-7 from China Medical University were in RPMI 1640 supplemented with 10% FBS, penicillin G (100 U/mL) and streptomycin (100 μg/mL).
Bacterial strains and plasmids used in this study were listed in Table 1, while the primers were listed in Table 2. S. aureus o165Z-1 and Escherichia coli strains were cultivated at 37 °C on Luria-Bertani (LB) medium. Kanamycin (40 μg/mL) and/or Isopropyl-β-D-thiogalactopyranoside (IPTG) (0.2 mmol/L) was added to the medium when needed. In all cases, the cultures were incubated in conical flask at 200 rpm (NBS, Series 25D, New Brunswick, USA).
Table 1 Bacterial strains and plasmids used in this study
1.2 DNA manipulation
All molecular manipulations were performed according to standard procedures[26]or those recommended by the manufacturers. All restriction endonucleases and Ex Taq DNA polymerase were purchased from TaKaRa (Dalian, China) and T4 DNA ligase from Promega (USA).
The full length SEC2-encoding gene was amplified from genomic DNA of S. aureus o165 through PCR using primers S1 and S2 (Table 2). A series of plasmids were constructed to express the translational products of the wild type and the mutated sec2 gene. Various C-terminus deletion SEC2 mutants were also obtained with a similar approach employing the primers P1, P2, P3, P4, and P5 and P7, respectively, serving as the 3′ end PCR primers instead of primer S2. N- and C-terminus deletion SEC2 mutants were also obtained with a similar approach employing the primers P2 and P6. Site-directed mutagenesis was performed using MutanBEST kit (TaKaRa, China). The DNA sequences were confirmed by TaKaRa (Dalian, China).

Table 2 Primers used in this study
1.3 Expression and purification of SEC2 mutants
Expression of SEC2 mutants were respectively induced with 0.2 mmol/L IPTG after growth for 4 h at 30 °C. Cells were harvested by centrifugation for 10 min at 4 °C and 8 000 r/min, and the cell pellets were resuspended in ice-cold buffer A (20 mmol/L Tris-HCl, pH 7.9, 0.5 mol/L NaCl). Cells were disrupted by sonication at 0 °C and centrifuged for 30 min at 4 °C and 15 000 r/min. The supernatants were collected and loaded onto the Ni+-NTA His·Bind Superflow column (Qiagen, Germany) equilibrated with buffer A. After nonspecifically bound proteins were washed off with buffer A containing 20 mmol/L imidazole, the specifically bound protein was eluted with buffer A containing imidazole from 50 to 250 mmol/L. The purity of the eluted protein was determined by SDS-PAGE[27].
1.4 Biological activity assay in vitro
Peripheral blood mononuclear cell (PBMC) proliferation and anti-tumor activities of NSM were determined by methyl thiazol tetrazolium (MTT) assay in vitro according to the method described previously[25]. PBMC from the blood of healthy donors was isolated by lymphocytes separation medium (TBD) and aliquoted to 1×105cells/ well in 96-well plate in RPMI 1640 (Hyclone) supplemented with 10% characterized FBS (TBD). Tumor cells were seeded in 96-well plate at a density of 1×104cells/ well in DMEM or RPMI 1640 supplemented with 10% FBS, respectively. Tumor growth inhibition (%) =100?[(the OD570of protein-treated cells well?the OD570of PBMC-releasing wells)/(the OD570of unsettled tumor cells control wells?the OD570of blank control wells)]×100. These data were presented as±s from three independent experiments and statistically analyzed by Student’s t-test.
The standard ferret feeding assay for SEs[28]was used for comparing emetic capability of SEC2 and NSM. Experiments were performed with adult female ferret with a mean body weight of 700 g.
Four to 6-week old healthy Kunming mice were purchased from China Medical University. Tumor cells (Cx-1 or MCF-7) were cultured to log phase, and diluted to concentration of 1×106cells/mL. Tumor cells (0.2 mL) were respectively injected into the right upper flank region of each mouse. After 24 h, they were weighted and randomized into 5 groups (n=6), including a normal control group without tumor injection, a group A with Cx-1 injection, a group B with MCF-7 injection, a group C that was injected with Cx-1 plus NSM at various dosages (20, 200, 500 ng/kg), a group D that was injected with MCF-7 plus NSM at various dosages (20, 200, 500 ng/kg). NSM was administrated daily by gavage for 7 days. On day 8, all mice were weighted and killed, and then the tumor was removed and weighted.
1.5 Sequence analysis
DNA sequence was analyzed using the software Vector NTI suite 8.0 (Informax, Invitrogen, USA). The binding of peptides to MCH II alleles were analyzed in the website of Center for biological sequence analysis of Technical University of Denmark DTU (http://www. cbs.dtu.dk/services).
1.6 Statistical analysis
Data were presented as means±standard errors of the mean (SEM). Statistical comparisons were performed using the one-way ANOVA and Student’s t test with SPSS software (SPSS, Germany), P<0.01were considered statistically significant.
2 Results
2.1 Truncation of SEC2 through PCR
To truncate SEC2 so as to obtain mutants possessing such merits as low molecular weight and anti-tumor activity but lacking of emetic activity, a series of plasmids harboring the mutated SEC2 gene were constructed through PCR and confirmed by DNA sequencing (Table 1). The mutant proteins produced in recombinant E. coli under IPTG induction were respectively purified through the Ni+-NTA His·Bind Superflow column and termed as SA1, SA2, SA3, SA4, SA5 and SA6 (Fig. 1). The purified proteins were analyzed by SDS-PAGE. The results showed that the purity of all mutants were greater than 95%.
2.2 PBMC proliferation activity of the truncated SEC2 mutants
The native SEC2 and truncated mutants were tested for their ability of stimulating PBMC proliferation. Dilutions of each protein were tested in triplicate wells. Results showed that nearly all mutated proteins exhibited PBMC proliferation activity similar to that of SEC2 other than SA6 (Fig. 2). In particular, SA2 possessed nearly the same PBMC proliferation activity as that of SEC2. It was interesting that SA2 with a deletion of C-terminal 131 amino acid residues still presented PBMC proliferation activity, suggesting that these 131 amino acids residues were not important for the T-cell stimulatory activity of SEC2. It was nearly the same with the published results[18].
Fig. 1 Truncation of SEC2 through PCR and the obtained mutants.
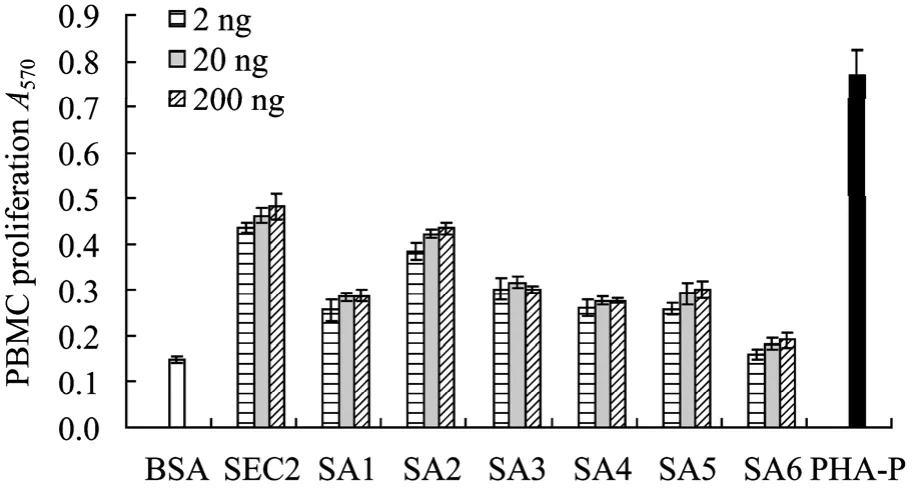
Fig. 2 PBMC proliferation assay of the SEC2 mutants by MTT assay. All values are averaged from five independent experiments, n=5,±s. Compared with negative control group: P<0.01.
The deletions of the 18 or 28 amino acids at N terminus of SEC2 significantly repressed the proliferactivity[18]. However, the deletions of the first 11 amino acids at the N-terminus had no effect on the ability of T-cell proliferation. To further low the molecular weight of SA2, the 17 amino acids at N terminus of SA2 was truncated through PCR, resulting the plasmid pSAG. Considering SH-group of Cys93 is active group, it may result in structural instability. Cys93 was replaced by Ala through site-directed mutagenesis. The obtained plasmid pNSM was confirmed by DNA sequencing. The mutant protein produced in recombinant E. coli harboring pNSM under IPTG induction was purified through the Ni+-NTA His·Bind Superflow column and termed as NSM. Production of NSM was checked through SDS-PAGE analysis by using whole-cell extracts. After purification, there was a single band on SDS-PAGE, showing that purity of NSM was more than 95% (Fig. 3).
Fig. 3 Expression and purification of protein. (A) The expression of the novel SEC2 mutant (NSM) induced with 0.2 mmol/L IPTG at 30 °C for 4 h. SDS-PAGE showed that the target protein with about 14 kDa could be detected in the lysate of E. coli BL 21 (DE3) harboring the plasmid pNSM. M: protein molecular marker (TaKaRa, China); 1: uninduced SEC2; 2: soluble expression of SEC2; 3: uninduced NSM. 4: induced NSM. (B) The purification of SEC2 and NSM through the Ni+-NTA His·Bind Superflow column (Qiagen, Germany) and Sephadex G-75. M: protein molecular marker (TaKaRa, China). 1: the purified SEC2; 2: the purified NSM.
2.3 Biological activity assay of the truncated SEC2 mutants in vitro
Biological activities of the native SEC2 and NSM were evaluated by MTT assay in vitro. Dilutions of each protein were tested in triplicate wells. Firstly, PBMC proliferation activity of NSM was examined, and BSA and PHA-P were respectively used as negative and positive control. Results showed that the stimulation of PBMC proliferation by NSM was very efficient even at the concentration of 2 ng/mL, which was equivalent to that of SEC2 (Fig. 4), indicating that the deletions of the 17 amino acids at SA2 N-terminus and site-directed mutagenesis of Cys93 did not affect PBMC proliferation activity. The effects of inhibition on the growth of tumor cells Cx-1 and MCF-7 induced by SEC2 and NSM were also determined. Results (Fig. 5) showed that inhibitory effects of SEC2 and NSM on tumor cells Cx-1 and MCF-7 were all in a dose-dependent manner, producing a significant anti-tumor activity at the concentration of 2 ng/well and a maximum effect at 500 ng/well. The results also showed that the mutant with the deletion of E1-F17 and T109-G239 as well as C93A exhibited nearly the same growth inhibition activity against tumor cells as SEC2. The further deletion of 17 amino acids from SA2 N-terminus and mutation of Cys93 did not affect anti-tumor activity in vitro compared with SEC2.
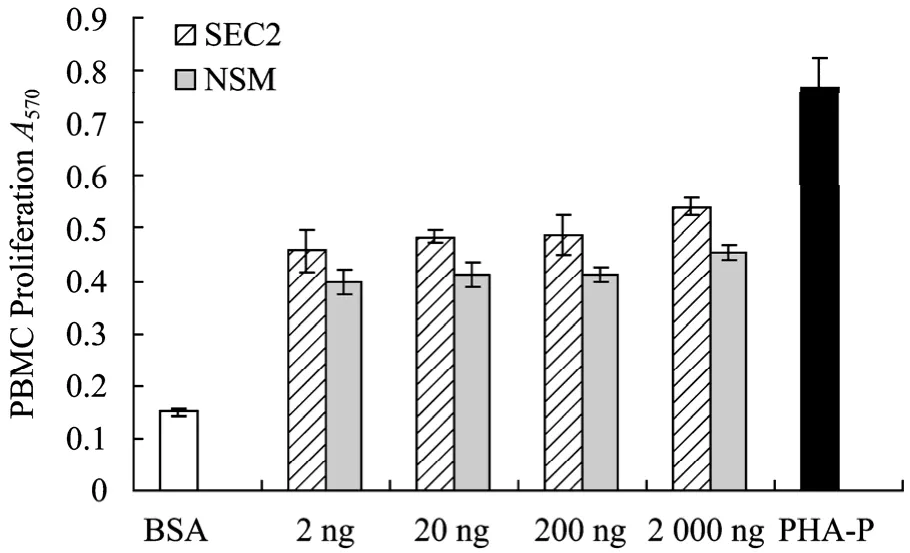
Fig. 4 PBMC proliferation assay of SEC2 and NSM by MTT assay. All values are averaged from five independent experiments, n=5,±s. Compared with negative control group: P<0.01.
Fig. 5 Anti-tumor activities of SEC2 and NSM by activating PBMC proliferation in vitro. All values are averaged from five independent experiments, n=5,±s. Compared with negative control group: P<0.01.
Secondly, the ability of NSM to inducing an emetic response was assessed in a Ferret model. The minimal emetic dose of SEC2 for ferret was 1 mg. In initial experiments, the emetic ability of NSM was assessed and administered at 10 mg to ensure an excess over the minimal emetic dose. Results showed that it no longer induced emetic response even if the dose represented a 10-fold excess of the amount of SEC2 required (Table 3). Indeed, the gold standard to assess emetic activity of SEs is oral administration to primates. Further experiment using primate model is needed to confirm loss of emetic activity of NSM.
Thirdly, the ability of NSM to inhibiting the tumor growth was assessed in tumor-bearing mice model. Results (Table 4) showed that NSM obviously inhibited the tumor growth in tumor-bearing mice. It was consistent with anti-tumor activities of NSM activating PBMC proliferation in vitro.
Table 3 Emetic response induced by NSM
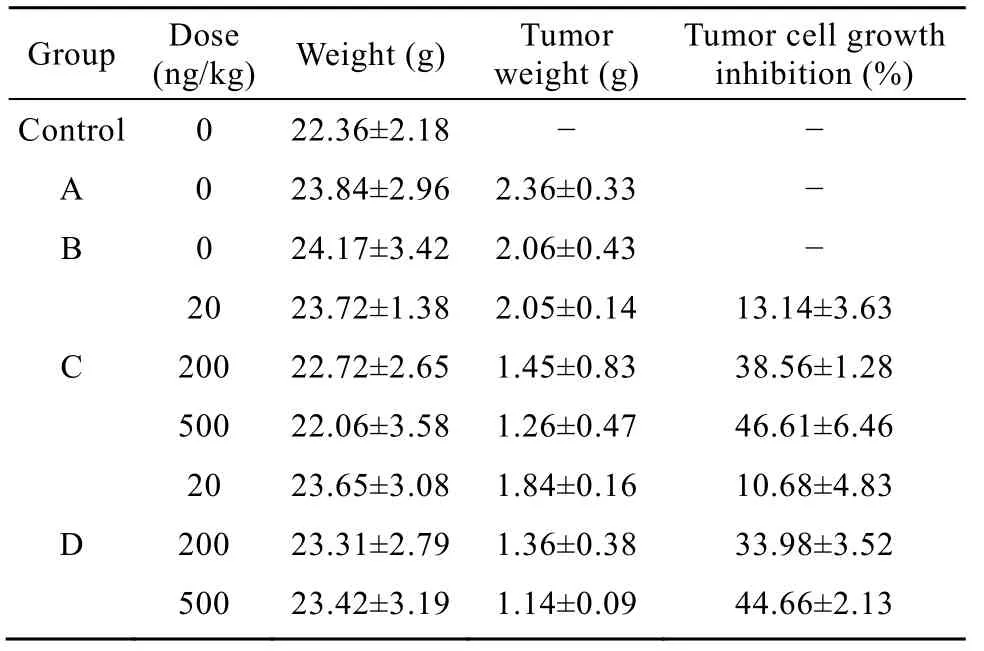
Table 4 Effects of NSM on the tumor growth in tumorbearing mice (n=6)
3 Discussion
SAg SEs deserved growing attention as ideal drugs for cancer therapy due to their ability to stimulate T cells at a high frequency, thereby giving rise to potent cell-mediated immunological responses and producing a large variety of cytokines inducing the final result of apoptotic death of tumor cells[29]. The SEC2 drug, termed as Gaojusheng in China, has been used to cure colorectal cancer, lung cancer, esophageal cancer, liver cancer, ovarian cancer, liver cancer, cancer of colon, leukemia, carcinoma of urinary bladder, et al, in clinic since 1994 and some encouraging results have been reported[30-31]. It proved that SEC2 was safe and effective. However, such side-effects as high molecular weight and emetic activity have restricted its clinical applications as agent of anti-tumor immunotherapy. In this paper, PCR and site-directed mutagenesis technology were utilized to truncate SEC2 so as to get the novel agent of anti-tumor.
SAgs are able to crosslink MHC class II molecules and TCR in a variety of subtly different ways through the various structural regions within each toxin[12,32-35]. The anti-tumor capabilities of SAg SEs depend on their MHC II binding ability[3,32]. T cell proliferation efficiency directly correlated with affinity for MHC class II[32]. Therefore, it is necessary to preserve the functional sites responsible for MHC II binding and T-cell stimulation. Until now, however, the regions responsible for SAg activity of SEs have not been completely confirmed. After A163-G239, G113-G239 and S87-G239 of SEC2 C-terminus were respectively deleted, the obtained mutants did not affect their superantigenic activity[18]. Results showed that the mutants, which were obtained after V91-G239 (SA1), T109-G239 (SA2), I115-G239 (SA3), Q129-G239 (SA4), R164-G239 (SA5) and S87-G239 (SA6) were respectively deleted from SEC2 C-terminus, still possessed T-cell stimulating activity. Particularly, SA2 exhibited nearly the same PBMC proliferation activity with that of SEC2, indicating that it preserved the specific regions and residues responsible for T-cell stimulatory activities. It was not completely consistent with the published results[18], which thought that the deletions of the last amino acids 113-239 affected superantigenic activity. Compared with crystal structure of SEA[13,36], SEB[37]and SEC2[11-12], the crucial functional sites responsible for binding MHC class II and TCR have been primarily confirmed. Residues of T109-G113 situated β5 region, it was important to insure crucial functional sites to form the particular three-dimensional structure, which was necessary to bind MHC class II and TCR. NSM, truncated 17 amino acids from SA2 N-terminus and replaced Cys93 with Ala, efficiently stimulated T cell proliferation, indicating that it preserved the affinity for MHC class II and stable structure. It was consistent with the sequence analysis of immunological features of NSM done in the website of http://www.cbs.dtu. dk/services. NSM possessed nearly the same MHC II binding alleles with SEC2. In addition, the deletion of 11 N-terminus residues of SEC2 had no effect on the T-cell stimulatory activity, and further deletions of 18 or more N-terminus residues severely impaired superantigenic activity[18]. Compared with the result, NSM still preserved T-cell stimulatory activity, indicating that the region of amino acids 1-17 of SEC2 did not particulate in MHC class II and TCR binding. However, T18 of SEC2 N-terminus was essential for MHC class II and TCR binding. It was consistent with the result obtained from crystal structure of SEC2[12-13].
One of the distinctive features of SAgs is their ability to cause proliferation of T cells and release massive cytokines, which produces significant tumor inhibition in vivo and in vitro[3,32]. NSM also exhibited nearly the same tumor-inhibition effects on Cx-1 and MCF-7 as SEC2, indicating that it possessed the distinctive feature of SAgs. It also proved that the proper three-dimensional structure of SEC2 was not required for its biological activity[18]. The fact that NSM lacking of the disulphide bond and cystine loop no longer induced emetic response strongly proved that the disulphide bond and cystine loop is not just an absolute requirement for lymphocyte but is related to emetic activity of SEs.
The problems for clinical application of superantigen protein drug are that proteins could not successfully permeate the biological barrier due to high molecular weight[10]and could result in emesis due to emetic activity of SEC2[38-39]. In this study, novel and no side-effect SEC2 mutant was constructed through deletion of the unnecessary residues which did not affect the T-cell stimulating activity and anti-tumor activity. The result presented in this study provided a possible strategy to solve the questions of protein drugs in clinic through deletion of unnecessary amino acids.
In conclusion, a desirable NSM was obtained. It is valuable for the further research and investigation of in vivo testing so as to provide novel agents with no side-effects and best treatment effects for clinic.
[1] Farser JD, Proft T. The bacterial superantigen and superantigen-like protein. Immunol Rev, 2008, 225(1): 226?243.
[2] Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science, 1990, 248(4956): 705?711.
[3] Pardoll DM. Parakrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol, 1995, 13: 399?415.
[4] Abrahmsén L. Superantigen engineering. Curr Opin Struc Biol, 1995, 5(4): 464?470.
[5] Alpaugh RK, Schultz J, McAleer C, et al. Superantigentargeted therapy: phase I escalating repeat dose trial of the fusion protein PNU-214565 in patients with advanced gastrointestinal malignancies. Clin Cancer Res, 1998, 4(8): 1903?1914.
[6] Hansson J, Ohlsson L, Persson R, et al. Genetically engineered superantigens as tolerable antitumor agents. Proc Natl Acad Sci USA, 1997, 94(6): 2489?2494.
[7] Hedlund G, Dohlsten M, Petersson C, et al. Superantigenbased tumor therapy: in vivo activation of cytotoxic T cells. Cancer Immunol Immunother, 1993, 36(2): 89?93.
[8] Mondal TK, Bhatta D, Biswas S, et al. Superantigeninduced apoptotic death of tumor cells is mediated by cytotoxic lymphocytes, cytokines, and nitric oxide. Biochem Biophys Res Commun, 2002, 290(4): 1336?1342.
[9] Ochi A, Migita K, Xu J, et al. In vivo tumor immunotherapy by a bacterial superantigen. J Immunol, 1993, 151(6): 3180?3186.
[10] Sharma P, Chawla HPS, Panchagnula R. The role of sorption promoters in increasing the bioavailability of drugs in oral preparations. Drugs Fut, 1999, 24(11): 1221?1240.
[11] Papageorgiou AC, Acharyal KR, Shapiro R, et al. Crystal structure of the superantigen enterotoxin C2 from Staphylococcus aureus reveals a zinc-binding site. Structure, 1995, 3(8): 769?779.
[12] Papageorgiou AC, Baker MD, Mcleod J, et al. Identification of a secondary zinc-binding site in staphylococcal enterotoxin C2: implications for superantigen recognition. J Biol Chem, 2004, 279(2): 1297?1303.
[13] Schad EM, Papageorgiou AC, Svensson LA, et al. A structural and functional comparison of staphylococcal enterotoxins A and C2 reveals remarkable similarity and dissimilarity. J Mol Biol, 1997, 269(2): 270?280.
[14] Spero L, Morlock BA. Biological activities of the peptides of staphylococcal enterotoxin C formed by limited tryptic hydrolysis. J Biol Chem, 1978, 253(24): 8787?8791.
[15] Hedlund G, Dohlsten M, Herrmann T, et al. A recombinant C-terminal fragment of staphylococcal enterotoxin A binds to human MHC class II products but does not activate T cells. J Immunol, 1991, 147(12): 4082?4085.
[16] Bohach GA, Schlievert PM. Conservation of the biologically active portions of staphylococcal enterotoxin C1 and C2. Infect Immun, 1989, 57(7): 2249?2252.
[17] Wahlsten JL, Ramakrishnan S. Separation of function between the domains of toxin shock syndrome toxin-1. J Immunol, 1998, 160(2): 854?859.
[18] Wang XG, Zhang HW, Xu MK, et al. Biological analysis of the deletion mutants of staphylococcal enterotoxin C2. Appl Microbiol Biotechnol, 2009, 83(6): 1077?1084.
[19] Antonsson P, Wingren AG, Hansson J, et al. Functional characterization of the interaction between the superantigen staphylococcal enterotoxin A and the TCR. J Immunol, 1997, 158(9): 4245?4251.
[20] Harris TO, Grossman D, Kappler JW, et al. Lack of complete correlation between emetic and T-cell-stimulatory activities of staphylococcal enterotoxins. Infect Immun, 1993, 61(8): 3175?3183.
[21] Hu DL, Omoe K, Saleh MHH, et al. Analysis of the epitopes on staphylococcal enterotoxin a responsible for emetic activity. J Vet Med Sci, 2001, 63(3): 237?241.
[22] Hoffmann ML, Jablonski LM, Crum KK, et al. Predictions of T-cell receptor- and major histocompatibility complex-binding sites on staphylococcal enterotoxin C1. Infect Immun, 1994, 62(8): 3396?3407.
[23] Hoffman M, Tremaine M, Mansfield J, et al. Biochemical and mutational analysis of the histidine residues of staphylococcal enterotoxin A. Infect Immun, 1996, 64(3): 885?890.
[24] Hovde CJ, Marr JC, Hoffmann ML, et al. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol Microbiol, 1994, 13(5): 897?909.
[25] Hui J, Cao Y, Zhang J, et al. Staphylococcus aureus enterotoxin C2 mutants: biological activity assay in vitro. J Ind Microbiol Biotechnol, 2008, 35(9): 975?980.
[26] Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd Ed. New York: Cold Spring Harbor Laboratory Press, 2001.
[27] Wang JZ, Fan M. Manual of Protein Technology. Beijing: Science Press, 2002.
[28] Wright A, Anderws PLR, Titball R. Induction of emetic, pyrexic, and behavioral effects of Staphylococcus aureus enterotoxin B in the ferret. Infect Immun, 2000, 68(4): 2386?2389.
[29] Sundstedt A, Celander M, Hedlund G. Combining tumor-targeted superantigens with interferon-alpha results in synergistic anti-tumor effects. Int Immunopharmacol, 2008, 8(3): 442?452.
[30] Chen TZ. The exploitation of HAS and its application in tumor therapy. Prog Microbiol Immuniol, 2001, 29: 63?69.
[31] Chen TZ. Gaojusheng: a novel anti-cancer drug prepared from SEC superantigen. Prog Microbiol Immunol, 2005, 33(2): 49?50.
[32] Baker MD, Acharya KR. Superantigens: structure-function relationships. Int J Med Microbiol, 2004, 293(7/8): 529?537.
[33] Jardetzky TS, Brown JH, Gorga JC, et al. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature, 1994, 369(6473): 711?718.
[34] Li HM, Llera A, Tsuchiya D, et al. Three-dimensional structure of the complex between a T cell receptor β chain and the superantigen staphylococcal enterotoxin B. Immunity, 1998, 9(6): 807?816.
[35] Swaminathan S, Furey W, Pletcher J, et al. Residues defining Vβ specificity in staphylococcal enterotoxins. Nat Struct Biol, 1995, 2(8): 680?686.
[36] Petersson K, Thunnissen M, Forsberg G, et al. Crystal structure of a SEA variant in complex with MHC class II reveals the ability of SEA to crosslink MHC molecules. Structure, 2002, 10(12): 1619?1626.
[37] Papageorgiou AC, Tranter HS, Acharya KR. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 ? resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J Mol Biol, 1998, 277(1): 61?79.
[38] Baker MD, Papageorgious AC, Titball RW, et al. Structural and functional role of threonine 112 in a superantigen Staphylococcal aureus enterotoxin B. J Biol Chem, 2002, 277(4): 2756?2762.
[39] Llewelyn M. Cohen J. Superantigens: microbial agents that corrupt immunity. Lancet Infect Dis, 2002, 2(3): 156?162.

