KCNQ1 rs2237895 gene polymorphism increases susceptibility to type 2 diabetes mellitus in Asian populations
Dong-Xu Li,Li-Ping Yin,Yu-Qi Song,Nan-Nan Shao,Huan Zhu,Chen-Sen He,Jiang-Jie Sun
Abstract BACKGROUND The association of single nucleotide polymorphism of KCNQ1 gene rs2237895 with type 2 diabetes mellitus (T2DM) is currently controversial.It is unknown whether this association can be gene realized across different populations.AIM To determine the association of KCNQ1 rs2237895 with T2DM and provide reliable evidence for genetic susceptibility to T2DM.METHODS We searched PubMed,Embase,Web of Science,Cochrane Library,Medline,Baidu Academic,China National Knowledge Infrastructure,China Biomedical Literature Database,and Wanfang to investigate the association between KCNQ1 gene rs2237895 and the risk of T2DM up to January 12,2022.Review Manager 5.4 was used to analyze the association of the KCNQ1 gene rs2237895 polymorphism with T2DM and to evaluate the publication bias of the selected literature.RESULTS Twelve case-control studies (including 11273 cases and 11654 controls) met our inclusion criteria.In the full population,allelic model [odds ratio (OR): 1.19;95% confidence interval (95%CI): 1.09-1.29;P < 0.0001],recessive model (OR: 1.20;95%CI: 1.11-1.29;P < 0.0001),dominant model (OR: 1.27.95%CI: 1.14-1.42;P < 0.0001),and codominant model (OR: 1.36;95%CI: 1.15-1.60;P=0.0003) (OR: 1.22;95%CI: 1.10-1.36;P=0.0002) indicated that the KCNQ1 gene rs2237895 polymorphism was significantly correlated with susceptibility to T2DM.In stratified analysis,this association was confirmed in Asian populations: allelic model (OR: 1.25;95%CI: 1.13-1.37;P < 0.0001),recessive model (OR: 1.29;95%CI: 1.11-1.49;P=0.0007),dominant model (OR: 1.35;95%CI: 1.20-1.52;P < 0.0001),codominant model (OR: 1.49;95%CI: 1.22-1.81;P < 0.0001) (OR: 1.26;95%CI: 1.16-1.36;P < 0.0001).In non-Asian populations,this association was not significant: Allelic model (OR: 1.06,95%CI: 0.98-1.14;P=0.12),recessive model (OR: 1.04;95%CI: 0.75-1.42;P=0.83),dominant model (OR: 1.06;95%CI: 0.98-1.15;P=0.15),codominant model (OR: 1.08;95%CI: 0.82-1.42;P=0.60.OR: 1.15;95%CI: 0.95-1.39;P=0.14).CONCLUSION KCNQ1 gene rs2237895 was significantly associated with susceptibility to T2DM in an Asian population.Carriers of the C allele had a higher risk of T2DM.This association was not significant in non-Asian populations.
Key Words: Type 2 diabetes mellitus;KCNQ1;rs2 237895;Single nucleotide polymorphism;Asian populations
lNTRODUCTlON
Type 2 diabetes mellitus (T2DM) is a common multifactorial,metabolic disease whose pathogenesis is influenced by a combination of genetic and environmental factors.The rise and large-scale application of genome-wide association studies have contributed to the understanding of genetic factors related to T2DM.T2DM remains a health problem that plagues the world to this day.As of January 4,2021,the number of people with diabetes worldwide had reached 537 million.Even more alarmingly,this number is expected to increase to 643 million by 2030.The various expenditures due to diabetes have exceeded $966 billion,and this figure has grown at an annual rate of 63% since 2006[1].The etiology of T2DM is complex and has not yet been fully elucidated.T2DM is characterized by defective insulin secretion and reduced sensitivity,leading to chronic hyperglycemia and severe metabolic dysfunction in patients[2,3].Hyperglycemia affects the physiological function of several tissues and organs in the body,among which the most common are neuropathy and vascular complications[1].
Studies have not provided an accurate description of the etiology of T2DM,and a genome-wide scan of Japanese by Nawataet al[4] showed thatKCNQ1is a susceptibility gene for T2DM in Japan.In addition,genes such asADRA2A,KCNJ11 and CDKAL1may be associated with the development of T2DM[4,5].KCNQ1is a potassium channel subunit that is mainly found in adipose and pancreatic tissues.It was found thatKCNQ1affects the process of islet β-cell depolarization by regulating potassium channel currents,thereby limiting insulin secretion from pancreatic β-cells and leading to the development of T2DM[6].
Previous studies have found that C allele carriers of theKCNQ1gene rs2237895 may have an increased risk of developing T2DM[7].rs2237895 is present in three genotypes in the population,AA,AC and CC.The A gene is wild type and the C gene is mutant,and their gene frequencies in the population are approximately 66% and 34%[8].A study by Cuiet al[7] in Kazakhs living in China showed that rs2237895 single nucleotide polymorphism (SNP) ofKCNQ1gene was not significantly associated with T2DM.A study by Afshardoostet al[9] on Iranians also showed no significant association between rs2237895 and T2DM;while in a study by Khanet al[10] on Indians,they confirmed a significant association between the SNP of rs2237895 and T2DM.Previously,a similar study has been conducted by Sunet al[11],but we consider that their inclusion criteria were more lenient and the strength of the proof may be weakened.Meanwhile,their work was > 10 years old and many new studies have been published during this period and that meta-analysis is in urgent need of updating.To address the above issues,we performed the present meta-analysis.
MATERlALS AND METHODS
Literature search
The following nine electronic databases were searched: PubMed,Embase,Web of Science,Cochrane Library,Medline,Baidu Academic,China National Knowledge Infrastructure (CNKI),China Biomedical Literature Database (CBM),and Wanfang Database,with the following search formulas: Subject (T2DM) and keywords (KCNQ1) and keywords (rs2237895).The last search date was January 12,2022.Chinese and English literature on the association of the rs2237895 SNP in theKCNQ1gene with T2DM was collected.The inclusion criteria for the articles were: (1) T2DM patients in the case group met the diagnostic criteria for diabetes published by WHO in 1999 or American Diabetes Association in 2010;(2) the type of experiment was a case-control study or a cohort study;(3) there was sufficient information in the text to describe the genotype and allele frequencies of the case and control groups;(4) the patients in the control group all met the Hardy-Weinberg genetic equilibrium model;(5) patients were randomly selected with no special restrictions on age,sex,or family history;and (6) for duplicate or data-identical literature,the one with the most complete information.Exclusion criteria were: (1) Incomplete study data;(2) literature reviews;(3) studies with gestational diabetes as an endpoint;and (4) exclusion of studies with familial diabetes as a basis.
Data extraction
Two researchers independently performed literature screening and extraction of information based on the above criteria.A third researcher was required to discuss and agree on the results when difficult differences were encountered.For each article,we collected the basic information that needed to be used for Meta-analysis,and the literature screening process is shown in Figure 1.

Figure 1 Literature screening process.
Statistical analysis
The data were processed using Review Manager 5.4.The strength of association between SNPs in theKCNQ1gene rs2237895 and the risk of T2DM was assessed using the odds ratio (OR) and its corresponding 95% confidence interval (95%CI) as a criterion in the data statistics.The forest plots were used to show the OR and its 95%CI for each study.Thepooled results were directly observed on the forest plots.The difference was considered significant when the 95%CI did not include 1.Allelic model (CvsA),recessive model (CCvsAA+AC),dominant model (CC+ACvsAA) and codominant model (CCvsAA and ACvsAA) were used to assess the genetic effects of the genes.The significance level was set atP< 0.05.The random-effect model was used to calculate the effect size when the heterogeneity wasI2> 50%,and the fixed-effect model was used whenI2was < 50%.Publication bias was assessed by Egger’s test and funnel plot.In the funnel plot,the dashed line perpendicular to the horizontal axis indicated the combined effect size.It suggested that the studies were without publication bias when the distribution of studies in the funnel plot was approximately symmetrical.
RESULTS
According to the research strategy,323 relevant papers were retrieved from the databases.Some duplicates were found and we removed them by Endnote software.We also screened the citations of the paper to ensure the comprehensiveness of the search.After a stepwise screening process,12 eligible papers were finally included for meta-analysis,which included 11273 patients with T2DM and 11654 controls.Five of the datasets were from China[12-16],five from the rest of Asia[7,10,17-19],one from Europe[20],and one from Africa[21].The basic information of the studies is shown in Table 1.

Table 1 Characteristics of the included literature
The 12 datasets that met the inclusion criteria were pooled for meta-analysis,and allelic,recessive,dominant,and codominant models were used to investigate the association of rs2237895 with T2DM.Since the study population was predominantly Asian,we performed stratified analysis of Asian and non-Asian populations (Figure 2 and Figure 3).

Figure 2 The forest plot of different model. A: Allelic model;B: Recessive model;C: Dominant model;D: Co-dominant model (CC vs AA);E: Co-dominant model (AC vs AA).T2DM: Type 2 diabetes mellitus.
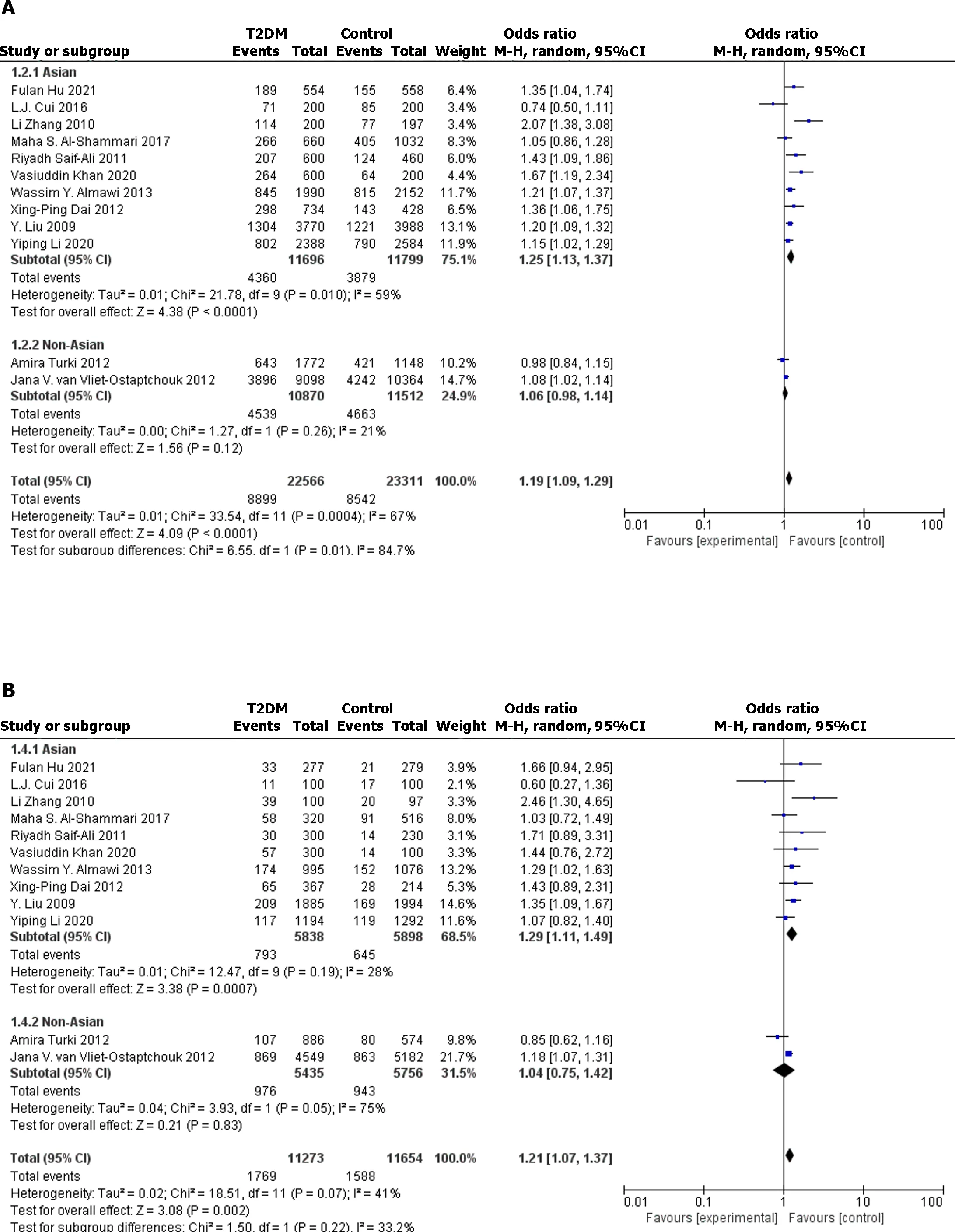
Figure 3 The forest plot for stratified analysis of different model. A: Allelic model;B: Recessive model;C: Dominant model;D: Co-dominant model (CC vs AA);E: Co-dominant model (AC vs AA).T2DM: Type 2 diabetes mellitus.
In the full population,allelic model (OR: 1.19;95%CI: 1.09-1.29;P< 0.0001),recessive model (OR: 1.20;95%CI: 1.11-1.29;P< 0.0001),dominant model (OR: 1.27.95%CI: 1.14-1.42;P< 0.0001),and codominant model (OR: 1.36;95%CI: 1.15-1.60;P=0.0003.OR: 1.22;95%CI: 1.10-1.36;P=0.0002) all showed significant association between rs2237895 and T2DM.In the subgroup of the Asian population,allelic model (OR: 1.25;95%CI: 1.13-1.37;P< 0.0001),recessive model (OR: 1.29;95%CI: 1.11-1.49;P=0.0007),dominant model (OR: 1.35;95%CI: 1.20-1.52;P< 0.0001),and codominant model (OR: 1.49;95%CI: 1.22-1.81;P< 0.0001.OR: 1.26;95% CI: 1.16-1.36;P< 0.0001) also showed a significant association between rs2237895 and T2DM,which was consistent with the whole population.C allele carriers had an increased risk of developing T2DM.The CC and AC genotypes significantly increased the risk of T2DM compared to the AA genotype.However,in the non-Asian population subgroup,allelic model (OR: 1.06;95%CI: 0.98-1.14;P=0.12),recessive model (OR: 1.04;95%CI: 0.75-1.42;P=0.83),dominant model (OR: 1.06;95%CI: 0.98-1.15;P=0.15),and codominant model (OR: 1.08;95%CI: 0.82-1.42;P=0.60) (OR: 1.15;95%CI: 0.95-1.39;P=0.14) all showed no significant association between rs2237895 and T2DM.
The funnel plots showed no significant publication bias was found in the meta-analysis (Figure 4 and Figure 5).Egger’s test showed no significant publication bias for the allelic model (t=1.84,P=0.095),recessive model (t=0.48,P=0.64),dominant model (t=1.44,P=0.18),and codominant model (t=1.33,P=0.21;t=1.79,P=0.10).

Figure 4 The funnel plot of different model. A: Allelic model;B: Recessive model;C: Dominant model;D: Co-dominant model (CC vs AA);E: Co-dominant model (AC vs AA).OR: Odds ratio.

Figure 5 The funnel plot for stratified analysis of different models. A: Allelic model;B: Recessive model;C: Dominant model;D: Co-dominant model (CC vs AA);E: Co-dominant model (AC vs AA).OR: Odds ratio.
We performed a sensitivity analysis.After sequentially excluding one study in the allelic model,recessive model,dominant model,and codominant model,we calculated the pooled effect sizes for the remaining studies.By calculation,no qualitative change occurred between the pooled results of the remaining studies and the original results.Sensitivity analysis proved that the results of the meta-analysis were reliable.
DlSCUSSlON
Compared with previous studies,we increased the inclusion criteria of cases,excluded the interference of other factors (e.g.,gestational diabetes),improved the strength of proof of the study,and made the results more reliable and stable.Our meta-analysis supported the findings of Khanet al[10],suggesting that the rs2237895 SNP in theKCNQ1gene is significantly associated with the development of T2DM in Asian populations.In the study by Cuiet al[7],the study population had an overall overweight problem,which increased the risk of T2DM prevalence and thus confounded the findings[22].
T2DM is a multifactorial,chronic,metabolic disease[23].The idea that genetic factors have a significant role in the development of T2DM is now more widely accepted[23],although only a few genes have been confirmed as a risk for the development of T2DM.However,many genetic characteristics associated with T2DM,such as effect sizes and risk allele frequencies,need to be explored[24].There is a need for researchers to identify risk genetic loci for T2DM and characterize the variation at the loci,thus providing a basis for elucidating the genetic pathogenesis of T2DM.
Previous studies have shown that theKCNQ1,miR-21,andArg972may be risk genes for T2DM[25,26].KCNQ1gene has now been shown to be located on chromosome 11p15.5,which is approximately 400 kb in length and consists of 17 exons ranging from 47 to 1122 bp in length[27].KCNQ1is associated with voltage-gated K+channels,and mutations in theKCNQ1gene lead to dysfunction of K+channels,which would cause diseases such as QT syndrome and familial atrial fibrillation.KCNQ1is expressed in many tissues[27,28],and the more studied about theKCNQ1gene is expressed in cardiac and pancreatic tissues[29].Current studies suggest that the main mechanisms of T2DM development are insulin resistance and islet β-cell dysfunction[2,23].Variants in theKCNQ1gene may lead to increased susceptibility to T2DM in the population by altering insulin secretion from pancreatic β-cells[30,31].It was hypothesized that variants in theKCNQ1gene would lead to increased expression ofKCNQ1protein on pancreatic β-cells,which in turn would alter the open state of voltage-gated potassium channels,decrease insulin secretion,and impair glucose storage and utilization[32].
This meta-analysis of theKCNQ1gene rs2237895 SNP and T2DM association study involved 12 studies,including 11273 T2DM patients and 11654 controls.This analysis showed that the rs2237895 polymorphism was significantly associated with an elevated risk of developing T2DM in an Asian population,which is consistent with Khanet al’s[10] findings.In Asian populations,C allele carriers have an increased risk of developing T2DM.The risk of T2DM is also increased in people with the CC and AC genotypes compared to the AA genotype.This is consistent with the previous findings of Huet al[16].Also,their findings showed that rs2237895 was associated with hypertension,body mass index,and hypertriglyceridemia.In non-Asian populations,this association was not significant.A 2015 study by Ríoset al[33] in Europeans also showed that theKCNQ1gene rs2237895 SNP was not significantly associated with T2DM,which is consistent with our findings.Our work provided strong evidence for the genetic pathogenesis of T2DM and helped to fully reveal the pathogenesis of T2DM.
This study showed that the rs2237895 SNP of theKCNQ1gene was differentially associated with T2DM in different populations.The reasons for this variation may be mutations in the regulatory region of theKCNQ1gene in particular populations[33],which interfere with the expression of theKCNQ1gene;or it may be due to the existence of different genotypes and allele frequencies in populations with different clinical characteristics,geographical distribution and ethnic origin;or differences in the external influences associated with T2DM,such as lifestyle and behavior,in different populations[4,23-25].The possibility of false-negative results in non-Asian populations with small study sample sizes cannot be excluded.
CONCLUSlON
In the Asian population,there was a significant association between theKCNQ1gene rs2237895 SNP and T2DM onset.C allele carriers were at increased risk of T2DM,and the CC and AC genotypes significantly increased the susceptibility to T2DM.However,in the non-Asian population,the association between rs2237895 and T2DM onset was not significant.
ARTlCLE HlGHLlGHTS
Research background
The association between the rs2237895 single nucleotide polymorphism (SNP) in theKCNQ1gene and the prevalence of type 2 diabetes mellitus (T2DM) has been controversial in different studies.
Research motivation
The aim of this study was to investigate the association between theKCNQ1gene rs2237895 and the prevalence of T2DM,and to provide help in establishing the pathogenesis of T2DM.
Research objectives
Demonstration of the association of the rs2237895 SNP in theKCNQ1gene with the prevalence of T2DM.Also,to explore whether this relationship differs in different populations.
Research methods
We searched nine databases.Two authors independently screened the literature according to the established inclusion and exclusion criteria.Finally,data extraction was performed and the data were meta-analyzed.
Research results
Twelve case-control studies met our inclusion criteria.After analysis,the rs2237895 SNP in theKCNQ1gene was associated with T2DM prevalence in Asian populations.However,this association was not significant in non-Asian populations.
Research conclusions
In Asian populations,carriers of the rs2237895 C allele of theKCNQ1gene were highly susceptible to T2DM compared to those who did not carry the C allele.However,in non-Asian populations,the association between the rs2237895 SNP and T2DM was not significant.
Research perspectives
We should continue to search for T2DM susceptibility genes through advanced technologies (e.g.,genome-wide association strategy) and gradually elucidate the pathogenesis of T2DM.
FOOTNOTES
Co-first authors:Dong-Xu Li and Li-Ping Yin.
Co-corresponding authors:Chen-Sen He and Jiang-Jie Sun.
Author contributions:Li DX,Yin LP,Sun JJ,and He CS designed this study (substantial contributions to the conception);Li DX,Yin LP,Song YQ,Shao NN,and Zhu H collected data;Li DX and Yin LP extracted and analyzed data,interpretation of data for the work;Sun JJ and He CS provided guidance for statistical analysis and provided financial support.They agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy;Li DX and Yin LP wrote the manuscript;Li DX,Yin LP,Song YQ,Shao NN,Zhu H,Sun JJ and He CS reviewed the manuscript;Li DX and Yin LP contributed equally to this work as co-first authors;Sun JJ and He CS contributed equally to this work as co-corresponding authors.The reasons for designating Li DX and Yin LP as co-first authors are as follows.First,Li DX and Yin LP contributed equal effort throughout the study.The selection of these researchers as co-first authors respects their equal contributions.Second,the research was conducted as a collaborative effort,and the designation of co-first authors accurately reflects the distribution of responsibilities and burdens associated with the time and effort required to complete the research and final paper.The reasons for designating Sun JJ and He CS as co-corresponding authors are as follows.First,Sun JJ and He CS put equal effort into the entire study.Second,the designation of co-corresponding authors best reflects the need for this study to have authors from different fields,which promotes the most in-depth examination of the research topic.In summary,we believe that the designation of Li DX and Yin LP as co-first authors and Sun JJ and He CS as co-corresponding authors meets the requirements of our manuscript,which reflects the spirit of equality and cooperation in our team.
Supported bythe Natural Science Foundation for the Higher Education Institutions of Anhui Province of China,No.2023AH050561,No.2022AH051143,No.KJ2021A0266,and No.KJ2021A1228;and School-level offline courses,No.2021xjkc13.
Conflict-of-interest statement:The authors declare no competing interests.
PRlSMA 2009 Checklist statement:The authors have read the PRISMA 2009 Checklist,and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Jiang-Jie Sun 0000-0002-8185-0802.
S-Editor:Lin C
L-Editor:A
P-Editor:Chen YX
 World Journal of Diabetes2024年3期
World Journal of Diabetes2024年3期
- World Journal of Diabetes的其它文章
- Regulatory role of peroxynitrite in advanced glycation end products mediated diabetic cardiovascular complications
- Effects of vitamin family members on insulin resistance and diabetes complications
- Chiglitazar and Thiazolidinedione in patients with type 2 diabetes: Which is better?
- Glucagon-like-peptide-1 receptor agonists and the management of type 2 diabetes-backwards and forwards
- Acute worsening of microvascular complications of diabetes mellitus during rapid glycemic control: The pathobiology and therapeutic implications
- Unlocking new potential of clinical diagnosis with artificial intelligence: Finding new patterns of clinical and lab data
