2-((2,6-二甲基苯基)氨基)乙醇鋁和鋅配合物的合成、結構及催化活性
劉安求 藺 隆 張德志 雷珺宇 王科峰 張 蔚*, 莊俊鵬 郝海軍*,
(1北京化工大學化學學院有機化學系,北京 100029)
(2中國石油天然氣股份有限公司石油化工研究院,北京 100195)
(3中國石油天然氣股份有限公司哈爾濱石化分公司,哈爾濱 150056)
Biocompatible polymers from lactides and lactones have received considerable attention and are extensively used in many fields owing to their environmentally friendly and biodegradable properties[1]. The aluminum[2-5]and zinc[6-8]complexes have attracted much interest as initiators for the ring-opening polymerization (ROP)of lactides and lactones to produce biodegradable polymers[9-10]due to their low toxicity, high reactivity, and inexpensiveness. Nevertheless, it has been well documented that the ligands govern the catalytic reactivity of the metal center in the complexes.Therefore,a variety of ligand systems (some are sophisticated) have been employed for aluminum and zinc complexes for ROP of cyclic esters, for example,half-salen[11-12]and salen-type[13-15], diphenol[16-17], Schiff base[18], ketiminate[18-19]andβ-diketiminate[7,20-21], bidentate [N,O][22-23]and [N,N][8,24]ligands, tridentate [N,N,N][25], [N,N,O][26-27]and [O,N,O][28], and [P,P,P][29]ligands. Our group has previously reported dimethylaluminum complexes based on bis(β- ketimininato)ligands as initiators for the ROP of lactones[30]. For the sake of the simplicity of the ligand systems, our attention is turned to the 2-(N-arylamino)ethanol[31-32]due to its readiness for synthesis, chelating nature, and variable steric hindrance of the ancillary aryl group. Herein, we report reactions of ArNHCH2CH2OH (HL, Ar=2,6-Me2C6H3) with AlMe3and ZnEt2and the catalytic activity of the resulted complexes in the ROP ofεcaprolactone.
1 Experimental
All manipulations of the moisture- and oxygensensitive organometallic compounds were carried out in a nitrogen atmosphere using the standard Schlenk technique or in a nitrogen-filled glove box.Hexane and toluene were dried by refluxing over sodium benzophenone ketyl and distilled in nitrogen. NMR spectra were obtained on a Bruker 400 MHz instrument and the chemical shifts were reported with respect to the reference (internal SiMe4for1H and13C NMR spectra). Elemental analyses were carried out on an Elementar Vario EL analyzer. The molecular weights of the polymers were determined on a Waters GPC515 - 2410 instrument using THF as the solvent.MnandMwvalues were determined from calibration plots established with polystyrene standards. AlMe3and ZnEt2were purchased from Sigma Aldrich and used as received.
1.1 Synthesis of HL
The ligand HL was synthesized by a modified procedure[32]. 2-Chloroethanol (8.9 g, 108 mmol), 2,6-dimethylaniline (13.2 g, 108 mmol), and potassium iodide (20 mg, catalytic amount) were added to a 100 mL round flask. The mixture was stirred for 3 h at 140 ℃and then cooled to room temperature to form a solid residue, which was recrystallized from ethanol to give colorless crystals of HL·HCl (12 g). The crystals were dissolved in 100 mL of water and neutralized with saturated NaHCO3solution, and the solution was extracted with dichloromethane (50 mL×3). The organic layers were combined, dried over MgSO4, and concentrated to give a colorless oil of HL (9.5 g, 53%).1H NMR (CDCl3):δ7.00 (d,J=6.6 Hz, 2H, C6H3), 6.85(t,J=6.6 Hz, 1H, C6H3), 3.75 (t,J=5.4 Hz, 2H, CH2O),3.12 (t,J=5.4 Hz, 2H, CH2NH), 2.75 (s, br, 2H, OH/NH),2.30(s,6H,CH3).
1.2 Synthesis of complex[(Me2Al)(L)]2(1)
HL (0.42 g, 2.55 mmol) and toluene (15 mL) were added to a 100 mL Schlenk flask. To this solution,AlMe3solution (2.06 g, 5.09 mmol, 17.8% in toluene)was syringed dropwise at room temperature. Bubbles were formed immediately. The reaction mixture was stirred at room temperature for 0.5 h. All the volatiles were removed in a vacuum and the residue was crystallized from a mixture ofn-hexane/toluene (1∶1,V/V) to give complex 1 as colorless crystals. Yield: 0.83 g(74%). m.p. 124-126 ℃.1H NMR (C6D6):δ7.19-6.89(m,6H,C6H3),3.55-3.53 (m,6H,CH2O/NH),2.77-2.75(t,J=7.2 Hz, 4H, CH2NH), 2.20 (s, 12H, C6H3(CH3)2),-0.40 (s, 12H, Al(CH3)2).13C NMR (C6D6):δ144.6,129.3, 129.3, 122.8, 61.6, 49.0, 18.9, -10.0. Anal.Calcd. for C24H40Al2N2O2(%): C 65.14, H 9.11, N 6.33;Found(%):C 64.86,H 8.78,N 6.05.
1.3 Synthesis of complex[EtZn2(L)3]2(2)
Complex 2 was similarly obtained as colorless crystals using ZnEt2solution (2 mol·L-1in toluene)instead of AlMe3solution as above-mentioned synthesis of 1.Yield:62%.m.p.115-117 ℃.1H NMR(CDCl3):δ7.03-6.98 (m, 12H, C6H3), 6.89-6.82 (m, 6H, C6H3),3.94-3.92 (m, 12H, CH2O), 3.58 (s, 4H, NH), 3.42 (s,2H, NH), 3.22-3.18 (m,12H, CH2NH), 2.32 (s, 36H,C6H3(CH3)2), -0.67 to -0.77 (m, 10H, ZnC2H5). Anal.Calcd. for C64H94N6O6Zn4(%): C 58.90, H 7.26, N 6.44;Found(%):C 58.67,H 6.94,N 6.19.
1.4 Crystal structure determination
Colorless crystals of 1 and 2 suitable for X-ray analysis were grown by slowly cooling their hotn-hexane/toluene solutions to room temperature under Ar.The N2 atom in 2 was found to be disordered.Satisfactory results were obtained when N2 was given an occupancy factor of 0.72 and N2A was given an occupancy factor of 0.28, respectively. All intensity data were collected on a Rigaku Saturn CCD detector using MoKαradiation (λ=0.071 073 nm) at 113 K. Semiempirical absorption corrections were applied using the CrystalClear program. The structures were solved by direct methods and difference Fourier map using SHELXS of the SHELXTL package and refined with SHELXL by full-matrix least-squares onF2.All nonhydrogen atoms were refined anisotropically. Hydrogen atoms were added geometrically and refined with riding model position parameters. A summary of the fundamental crystal data for these complexes is listed in Table 1.

Table 1 Crystallographic data and refinement parameters for complexes 1 and 2
CCDC:2304200,1;2304201,2.
1.5 General procedure for ε-caprolactone polymerization
Complex 2 (1.10 mL, 0.088 4 mol·L-1in toluene)was added to 15 mL of anhydrous toluene as an initiator. Then the calculated amount ofε-caprolactone was added by a syringe. The reaction mixture was stirred at 18 or 60 ℃for a specified time and quenched with two drops of acetic acid/water solution (1∶1,V/V). Hexane(20 mL) was added to the reaction mixture to precipitate the polymers, which were then filtered, washed with hexane, driedin vacuoto a constant weight, and analyzed by gel permeation chromatography(GPC).
2 Results and discussion
2.1 Synthesis of complexes 1 and 2
Treatment of HL with two equivalents of AlMe3or ZnEt2in toluene at room temperature gave complexes 1 and 2 in good yields (Scheme 1). It is noteworthy that complex 2 was also isolated even when one equivalent of ZnEt2was used. These two complexes are sensitive to moisture and oxygen, and soluble in common solvents, such as toluene, chloroform, and THF, but only sparsely soluble in hexane. Compared with the1H NMR spectrum of HL, the considerable changes of complexes 1 and 2 in their1H NMR spectra were the disappearance of the signal of proton attributed to the hydroxyl group and the appearance of the signals of hydrogens attributed to metal-alkyls (δ=-0.40 in 1 and-0.67 to -0.77 in 2), which illustrates the formation of the complexes.
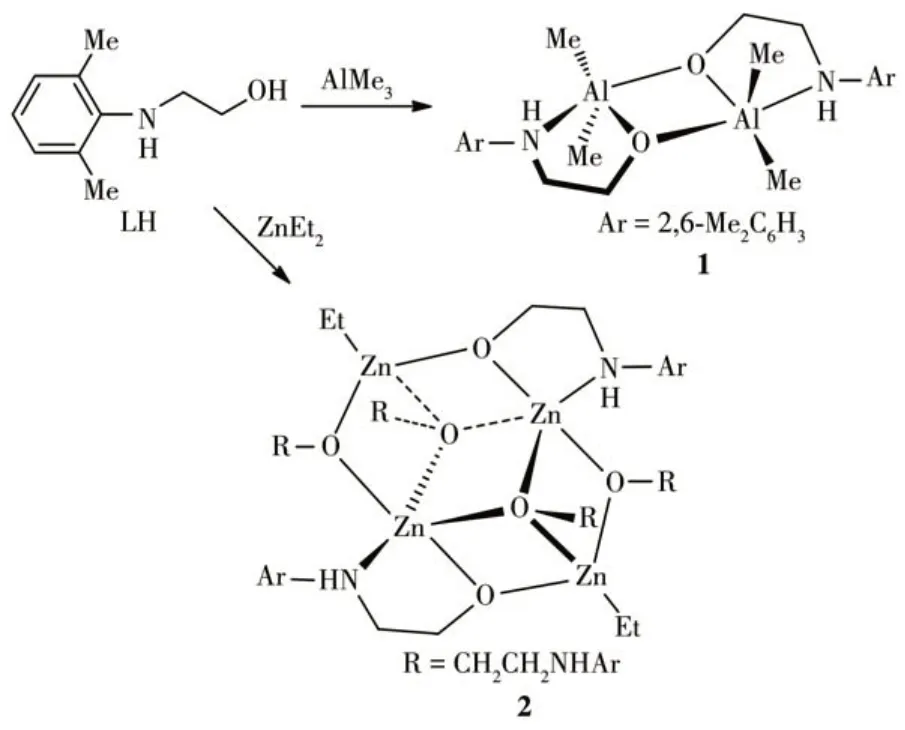
Scheme 1 Synthesis of complexes 1 and 2
2.2 Crystal structures of complexes 1 and 2
The molecular structures of complexes 1 and 2 were determined by single-crystal X-ray diffraction method, respectively. Their selected bond distances and angles are listed in Table 2.Fig.1 shows that complex 1 is a crystallographically centrosymmetric dimer in the solid state with an inversion center located at the center of the parallelogram of O1-Al1-O1A-Al1A, and each aluminum atom is chelated by the aminoethanolate ligand and bridged by the two oxygen atoms of ligands, resulting in the aluminum atom with a fivecoordinated environment. The Al1—O1 bond distance(0.184 6(2) nm) is shorter than Al1—O1A (0.192 0(2)nm). These values are similar to the Al—O distances in other five-coordinated aluminum compounds with an analogous Al2O2core, such as {[OCMe2CH(Me)=NAr]AlMe2}2(0.184 9(1) nm and 0.196 2(1) nm, Ar=2,6-diethylphenyl)[20]. The bond angles of O1—Al1—O1A and Al1—O1—Al1A are 76.76(7)° and 103.24(7)°,respectively. The O1, Al1, N1, and C2 atoms are almost coplanar with a mean deviation of only 0.001 42 nm, and the C1 atom is deviated from this plane at 0.059 19 nm. The dihedral angle of the O1—Al1—N1—C2 plane and the O1—Al1—O1A—Al1A plane is only 1.4°, which means that the Al2O2core structure of complex 1 is almost planar. The two methyl groups connected to the Al atom are inequivalent because of the rigid structure. However, the two methyl groups display as a singlet in the1H NMR spectrum, suggesting that, in CDCl3solution, quick inversion of the configuration of nitrogen takes place at room temperature due to its weak coordination with the aluminum atom.This is evidenced by the Al1—N1 distance (0.237 8(2)nm), which is longer than the Al—N bond distance in{[OCMe2CH(Me)=NAr]AlMe2}2(0.220 7(1)nm)[20].
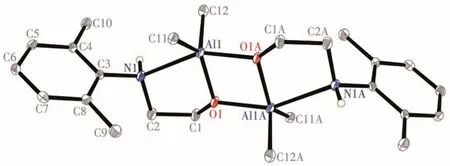
Fig.1 Molecular structure of complex 1 with 30%probability displacement ellipsoids

Table 2 Selected bond distances(nm)and angles(°)for complexes 1 and 2
Fig.2 displays that complex 2 is an interesting tetra-zinc aggregate with a centrosymmetric skeleton.The aminoethanolate ligands show three kinds of coordination modes in this complex. The N1 and O1 atoms from one ligand are coordinated to Zn1 to form a fivemembered ring,meanwhile,the O1 atom is also coordinated to Zn2A. The O3 atom from another ligand is coordinated to Zn1 and Zn2 at the same time, and the N3 atom from this ligand is non-coordinated. In addition, the O2 atom from the third ligand is coordinated with Zn1, Zn2, and Zn1A atoms from the top of the plane of Zn1-Zn2-Zn1A-Zn2A, and the N2 atom from this ligand is also non-coordinated. Since the structure of 2 is complicated,the Zn4O6core structure of 2 is presented in Fig.3. The Zn4O6core can be viewed as two side-by-side cubes, sharing the O2-Zn1-O2A-Zn1A face and missing a pair of opposite vertices.The coordination sphere of Zn1 is best described as pyramidal geometry with O1-O2-O3-N1 as the square base and O2A as the vertex, while that of Zn2 is tetrahedral geometry. The Zn—O bond distances vary from 0.195 2 to 0.213 8 nm, comparable to those found in other polynuclear zinc compounds, such as {[ArN=C(Me)COCHCO(Me)C(Me)=NAr] [OCH(Me)C(Me)=NAr] (ZnEt)3} (0.196 7(3)-0.209 5(3) nm, Ar=2, 6 -iPr2C6H3) with a Zn3O3core[20]and (S)-diphenyl(pyrrolidin-2-yl)methanolate ethylzinc (B) with a Zn2O2core(0.202 4(2)-0.203 8(2) nm)[33]. The Zn1—N1 bond distance is 0.223 9(3) nm, slightly longer than those in B(0.213 6(2) and 0.215 6(2) nm)[33]. The bond angles of Zn2-O2-Zn1A and Zn1—O2—Zn1A are 94.99(9)° and 92.79(8)°,respectively.
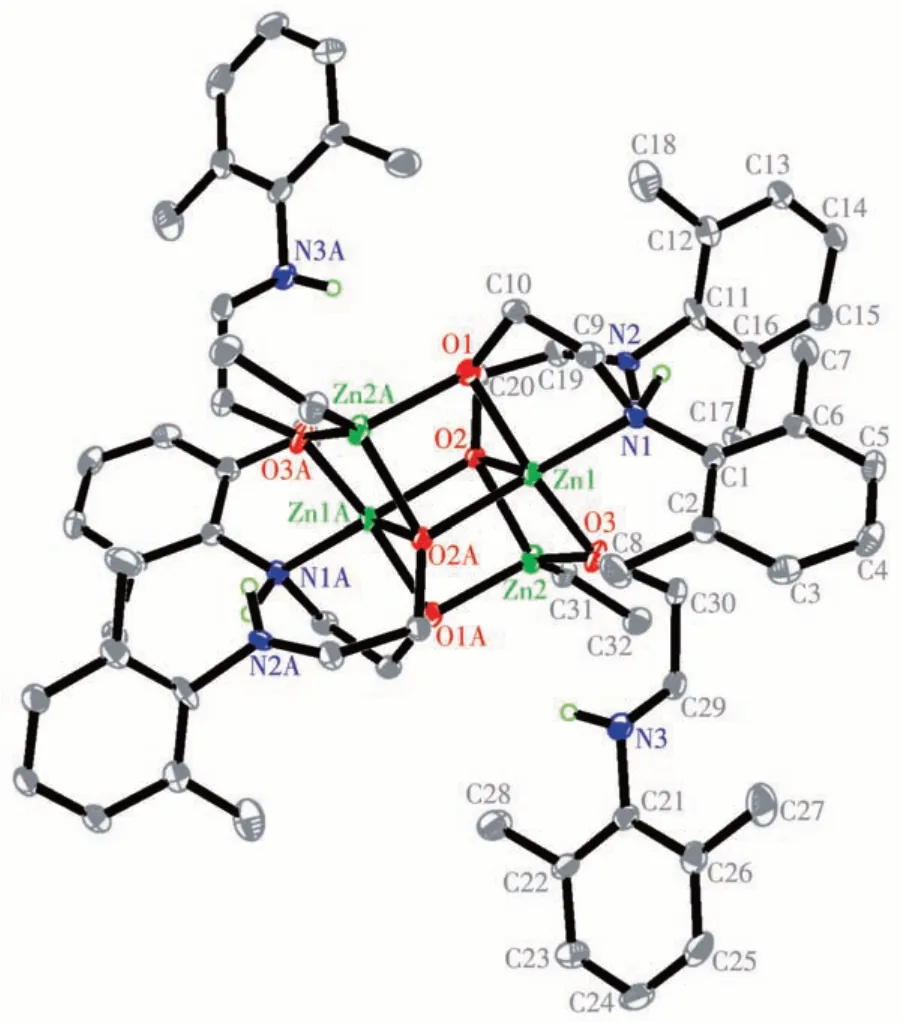
Fig.2 Molecular structure of complex 2 with 30%probability displacement ellipsoids

Fig.3 Core structure of complex 2 with 30%probability displacement ellipsoids
2.3 Catalytic activity of complexes 1 and 2
The aluminum or zinc-catalyzed ROP ofε-caprolactone has been widely investigated in recent years[2-3]. It′s known that the ligands significantly change the catalytic reactivity of the metal center.Herein, the ROP ofε-caprolactone catalyzed by complexes 1 and 2 have also been examined. Complex 1 showed negligible activity toward the ROP ofε-caprolactone. Similar results have been observed in other dimeric aluminum complexes with an Al2O2core,which seems that the dimeric structures disfavor the initiation of the ROP ofε-caprolactone[23]. In contrast,the zinc complex 2 showed good activity in this reaction. The results of the ROP ofε-caprolactone in toluene catalyzed by complex 2 at 18 and 60 ℃are listed in Table 3. In general, higher temperature results in a shorter reaction time with higherand yield (entries 3-6). When the molar ratio of the initial monomer (CL)to the initiator (I),nCL/nI, was increased from 200 to 1 600, thewas increased from 3 721 to 28 617,whereas the yield of the poly(caprolactone) was decreased from 93% to 79% after the set reaction time.The measuredvalues ranged from 1.20 to 1.34 whennCL/nIexceeded 400, which were comparable to those of the recently reported Zn complexes[34]. Thedetermined from GPC was slightly less than one-fourthof that calculated fromMn(Calcd.), suggesting that all of the four zinc atoms of 2 are active centers.

Table 3 ROP of ε-caprolactone catalyzed by complex 2
3 Conclusions
In summary, the aluminum and zinc complexes chelated by 2-((2,6-dimethylphenyl)amino)ethanolate have been synthesized and characterized. Preliminary catalytic studies prove that the zinc complex 2 is highly active toward the ring-opening polymerization ofε-caprolactone, giving poly(caprolactones) with high molecular weights and narrow molecular weight distribution, while the aluminum complex 1 is inactive. This suggests that appropriately combining metal and simple ligands can also lead to a good catalyst.

