柑橘衰退病毒RT-RPA-LFD可視化檢測方法的建立及應用
申世凱 曾婷 喬興華 陳力 任杰群 周彥
摘? ? 要:【目的】柑橘衰退病由柑橘衰退病毒(citrus tristeza virus,CTV)引起,是一種世界性的重要柑橘病害。為實現CTV的田間快速檢測,建立一種準確、快速且可視化的檢測方法。【方法】以CTV外殼蛋白(CP)的保守區域為靶標,設計3對特異性引物和探針,通過引物篩選,以及優化引物濃度、反應時間和反應溫度等條件,建立CTV的反轉錄-重組酶聚合酶擴增-側流層析試紙條(RT-RPA-LFD)快速檢測方法,明確其靈敏度,并用于田間疑似樣品的檢測。【結果】建立了CTV的RT-RPA-LFD檢測方法:最佳檢測引物為RPA-1F/R,對應探針為RPA-P,最佳反應條件為40 ℃,25 min,且與其他5種柑橘病毒無交叉反應。該方法的靈敏度是RT-PCR的100倍,最低可檢測到2.12×101 拷貝·μL-1的CTV核酸,與RT-qPCR相當。采用RT-RPA-LFD法在67份田間樣品中檢測出CTV陽性樣品41份,與RT-PCR法檢測結果一致。【結論】建立的CTV RT-RPA-LFD法具有操作簡單、快速、結果可視等優點,適合基層植保工作者對田間樣品開展快速檢測。
關鍵詞:柑橘;柑橘衰退病毒;反轉錄-重組酶聚合酶擴增-側流層析試紙條;快速檢測
中圖分類號:S666 文獻標志碼:A 文章編號:1009-9980(2023)12-2652-09
收稿日期:2023-07-28 接受日期:2023-10-20
基金項目:財政部和農業農村部國家現代農業產業技術體系(CARS-26-05B)
作者簡介:申世凱,女,在讀碩士研究生,研究方向為植物病理學。E-mail:1091893469@qq.com
*通信作者 Author for correspondence. E-mail:zybook1@163.com
Establishment and application of RT-RPA-LFD visualization assay for rapid detection of citrus tristeza virus
SHEN Shikai1, ZENG Ting1, QIAO Xinghua2, CHEN Li2, REN Jiequn3, ZHOU Yan1*
(1Southwest University, National Citrus Engineering Research Center, Chongqing 400712, China; 2Plant Protection and Fruit Technology Extension Station of Wanzhou District, Chongqing 400712, China; 3Chongqing Three Gorges Academy of Agricultural Sciences, Chongqing 400712, China)
Abstract: 【Objective】 Tristeza caused by citrus tristeza virus (CTV) is one of the most destructive citrus diseases in the world, which is mainly spread by several aphid species and bud-grafting. Severe CTV isolates could cause quick decline of sour orange rootstock, and stem pitting of susceptible cultivars. In recent years, stunted, severe stem pitting and reduced fruit quality were observed in Newhall navel orange and some tangor cultivars, causing severe economic losses in major citrus-growing provinces of China, especially in Hunan, Jiangxi, Yunnan, Sichuan provinces. Prompt and accurate CTV detection in the nursery and field samples is necessary to control CTV. To date, serological techniques, reverse transcription PCR (RT-PCR), RT- real-time PCR (RT-qPCR) and other methods have been used to detect CTV. However, these traditional detection techniques are generally flawed. The purpose of this study was to establish a reliable, accurate, convenient and visual reverse transcription-recombinase polymerase amplification (RT-RPA) combined with lateral flow dipstick (LFD) method for CTV detection. 【Methods】 Three pairs of primers and a specific probe used for CTV detection were designed according to the conservative sequence of the coat protein (CP) gene of CTV isolates (NCBI number MH558665.1, MH558666.1, JX266712.1, JQ911664.1 and JQ061137.1) from China. By detecting CTV-infected citrus samples, primers with the best specificity and amplification efficiency were selected to establish the RT-RPA-LFD for CTV detection. The total RNAs were extracted from 100 mg CTV-infected citrus leaf samples using RNAiso Plus and used for CTV detection. The reverse transcription was performed using a C1000 Thermal Cycler in a 20 μL reaction mix containing 1 μL of Oligo dT Primer, 1 μL of 10 μmol·L-1 dNTP Mixture, 1 μL of RNA template, 4 μL of PrimeScript Buffer, 0.5 μL of RNase Inhibitor, and 1 μL of PrimeScript RTase. The reaction was carried out for 45 min at 42 ℃ and 5 min at 95 ℃. RT-RPA-LFD reaction system was optimized with respect to the primer concentration (1, 2.5, 5, 10, 20, and 50 nmol·L-1), reaction time (5, 10, 15, 20, 25, 30, 35 and 40 min), and reaction gradient temperature (10, 15, 20, 25, 30, 35, 40, 45 and 50 ℃). For visual detection, LFD strips from the AmplifyRP × RT Discovery Kit were added to the RT-RPA products. The reactions should be allowed to incubate for no more than 30 min. The two visual bands of the test and control lines suggested that the tested sample was CTV-positive, and only one band on the control line indicated a negative result. The optimized reaction conditions were determined through the colour density of the test line. The specificity of the established RT-RPA-LFD was evaluated by detecting the samples infected with CTV, citrus yellow vein clearing virus (CYVCV), Citrus tatter leaf virus (CTLV), citrus exocortis viroid (CEVd), citrus psorosis virus (CPV), citrus chlorotic dwarf-associated virus (CCDaV), and the virus-free citrus plants, respectively. To evaluate the detection range of the optimized RT-RPA-LFD, eight CTV genotypes and eleven CTV isolates from different countries were used. A series of 10-fold dilutions (2.12×106-2.12×10-1 copies·μL-1) of CTV samples were used to test the sensitivity of the RT-RPA-LFD assay, and the sensitivity was compared with the conventional RT-PCR and RT-qPCR. Furthermore, the leaves of 67 CTV-suspected different tangor cultivar samples were randomly collected from Chongqing, Sichuan and Guangxi provinces, and used for RT-PCR and RT-RPA-LFD detection. 【Results】 A RT-RPA-LFD assay for rapid visual detection of CTV was established, with primer pairs RPA-1F (5-CTTGCTGGCGTCCCTTGTTTCTGTTCTTGTCTT-3) and RPA-1R (5-ATTCTGTTTCCTT TCCTAGCCGGGCTTCTTCAC-3), and RPA-P probe (5-GGCGAAAAATCTTTTCGTCTACT TGGTTTTCACTCGCGAAG GCA-3). It could specifically amplify the target fragment of CTV with a size of 156 bp. The optimal reaction conditions for the determination of RT-RPA-LFD assay were determined as 10 μmol·L-1 primer concentration, 25 min reaction time and 40 ℃ incubation temperature. This method has high specificity to CTV, and no test line was observed when total nucleic acid extracts from CTLV, CYVCV, CEVd, CPV, CCDaV, or healthy citrus plants were tested. This method could also detect different genotypes and origin of CTV. In the sensitivity detection, 2.12×101 copies·μL-1 was the lowest detection sensitivity of RT-RPA-LFD and RT-qPCR. The limit of detection of RT-PCR was 2.12×103 copies·μL-1, indicating that the RT-RPA-LFD method would be 100 times more sensitive than RT-PCR, which was consistent with that of RT-qPCR. Furthermore, the RT-RPA-LFD detection of CTV required shorter detection time (approximately 30 min) than RT-PCR and RT-qPCR. Among 67 citrus samples randomly collected from the field, CTV was detected from 41 samples using RT-RPA-LFD and RT-PCR assay showed the same results. These results suggested that the RT-RPA-LFD method would be suitable for CTV detection in the field. 【Conclusion】 In this study, a visual RT-RPA-LFD method for CTV detection was developed and the optimal reaction conditions for the RT-RPA-LFD assay were determined. The new RT-RPA-LFD method would be more effective and sensitive for the precise quantification of CTV than RT-PCR. It could be applied to on-site rapid detection for the plant protection and quarantine station.
Key words: Citrus; Citrus tristeza virus (CTV); Reverse transcription-recombinase polymerase amplification-lateral flow dipstick test strip; Rapid detection
柑橘衰退病是危害柑橘產業的重要病害之一,其病原為柑橘衰退病毒(citrus tristeza virus,CTV),主要通過感病接穗、苗木和多種蚜蟲進行傳播,廣泛分布于世界各柑橘產區[1]。CTV是長線性病毒科(Closteroviridae)長線性病毒屬(Closterovirus)的正義單鏈RNA病毒,基因組全長19.8 kb,含12個開放閱讀框(ORF),可編碼兩個外殼蛋白(CP和CPm),其中CP在CTV基因組中高度保守[2]。CTV在田間存在復雜的株系分化現象,除導致酸橙及其作砧木植株的快速死亡外,還導致葡萄柚、梾檬和部分甜橙、柚類、雜柑等敏感品種的莖陷點癥狀,以及酸橙、尤力克檸檬和葡萄柚實生苗的矮縮、黃化,CTV弱毒株在植株上不會產生嚴重的癥狀[3]。根據CTV的生物學特性及其基因組變異,CTV被分為T36、VT、T30、T3、RB、T68、HA16-5和S1等多個基因型,且其基因型的種類還在不斷增加[4-5]。
我國由于長期使用枳、香橙、酸柚等抗速衰型衰退病砧木,莖陷點型衰退病是我國衰退病危害的主要類型[6-7]。近年來,由于莖陷點型衰退病隨苗木流通,其發生范圍不斷擴大,已在湖南、江西、云南等柑橘主產區造成了嚴重的危害[8-10]。目前采用無毒繁殖材料是防治CTV最有效的手段,而這依賴于高效快速的檢測方法。
目前常用血清學[11]、RT-PCR[12]以及RT-qPCR[13-14]等方法檢測CTV。這些技術雖然靈敏度高、特異性強,但難以實現田間現場檢測。因此為滿足果園、苗圃現場快速檢測的需要,亟待研發一種準確、快捷、簡便的CTV檢測方法。重組酶聚合酶擴增(Recombinase polymerase amplification,RPA)模擬T4噬菌體核酸復制機制,在體外實現恒溫擴增[15]。通過結合側流層析試紙條(lateral flow dipstick,LFD),從而實現了檢測結果的可視化。由于RT-RPA-LFD檢測技術具有快速、靈敏和簡便的優點,尤其適用于普通工作人員開展田間檢測,目前已成功應用于柑橘碎葉病毒(citrus tatter leaf virus,CTLV)、櫻桃病毒A(cherry virus A,CVA)、李矮縮病毒(prune dwarf virus,PDV)、李痘病毒(plum pox virus,PPV)等多種植物病毒的檢測[16-19]。筆者在本研究中以CTV保守的CP基因為靶標設計特異性引物和探針,建立、優化了CTV的RT-RPA-LFD檢測技術,為CTV的快速檢測提供了新的選擇。
1 材料和方法
1.1 試驗材料
單一感染CTV、柑橘黃脈病毒(citrus yellow vein clearing virus,CYVCV)、柑橘碎葉病毒、柑橘裂皮病類病毒(CEVd)、柑橘鱗皮病毒(citrus psorosis virus,CPV)和柑橘褪綠矮縮病毒(citrus chlorotic dwarf-associated virus,CCDaV)的病株,無病毒柑橘植株;核酸濃度為2.12×106 拷貝·μL-1的CTV陽性樣品。以上材料均為西南大學柑桔研究所保存提供。
1.2 主要試劑
RPA擴增試劑盒購自美國Agdia公司;PlantGen DNA Kit購自中國康為世紀;RNAiso Plus試劑盒購自寶生物工程(大連)有限公司;All-In-One 5× RT MasterMix,2 × Taq Master Mix購自諾唯贊公司。
1.3 總核酸提取和cDNA合成
使用PlantGen DNA Kit和RNAiso Plus提取總核酸,并于-80 ℃冰箱保存備用。將1 μL Oligo dT Primer,1 μL dNTP Mixture(10 μmol·L-1),1 μL總RNA模板,7 μL ddH2O混合后,65 ℃ 5 min;冰上冷卻后加入4 μL PrimeScript Buffer,0.5 μL RNase Inhibitor(40 U·μL-1),1 μL PrimeScript RTase(200 U·μL-1,TaKaRa),加ddH2O至總體積為20 μL。42 ℃ 45 min,95 ℃ 5 min。
1.4 RT-RPA-LFD的引物和探針設計
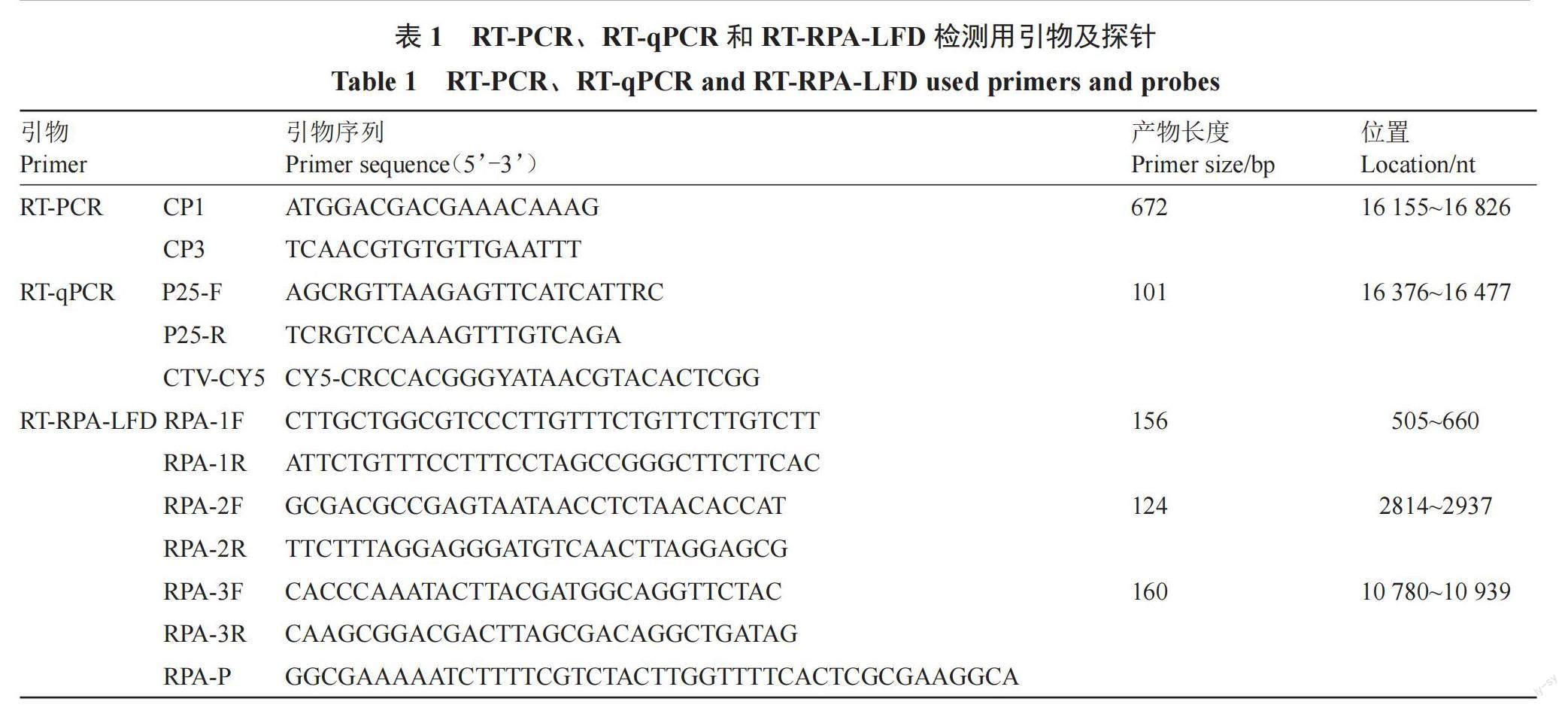
比對分析NCBI中已報道的5個中國莖陷點型CTV CP基因序列(GenBank:MH558665.1、MH558666.1、JX266712.1、JQ911664.1和JQ061137.1),以及Amplify Discovery Kits中引物和探針設計要求,使用Primer Premier 5.0設計特異性引物和探針(表1),所用引物和探針均由北京擎科生物技術有限公司合成。
1.5 RT-RPA-LFD反應體系的建立及優化
使用AmplifyRP × RT Discovery Kit進行RPA擴增反應。反應體系包括5.9 μL Rehydration Buffer,0.42 μL RPA-F/R(設6個濃度梯度:1.0、2.5、5.0、10.0、20.0、50.0 μmol·L-1)、10 μmol·L-1 RPA-P 0.12 μL、1 μL cDNA、1.64 μL ddH2O。反應液與固體反應物混勻后加入0.5 μL 280 mmol·L-1 MgOAc進行孵育(分別設置8個反應時間5、10、15、20、25、30、35、40 min和9個溫度梯度10、15、20、25、30、35、40、45、50 ℃)。孵育結束后,放入試紙條,室溫放置10~20 min后觀察結果。以質控線(Control line)和測試線(Test line)顯示清晰,判斷結果為陽性;質控線顯示清晰,測試線無條帶時,結果為陰性;質控線未出現條帶時,結果無效。
1.6 特異性分析
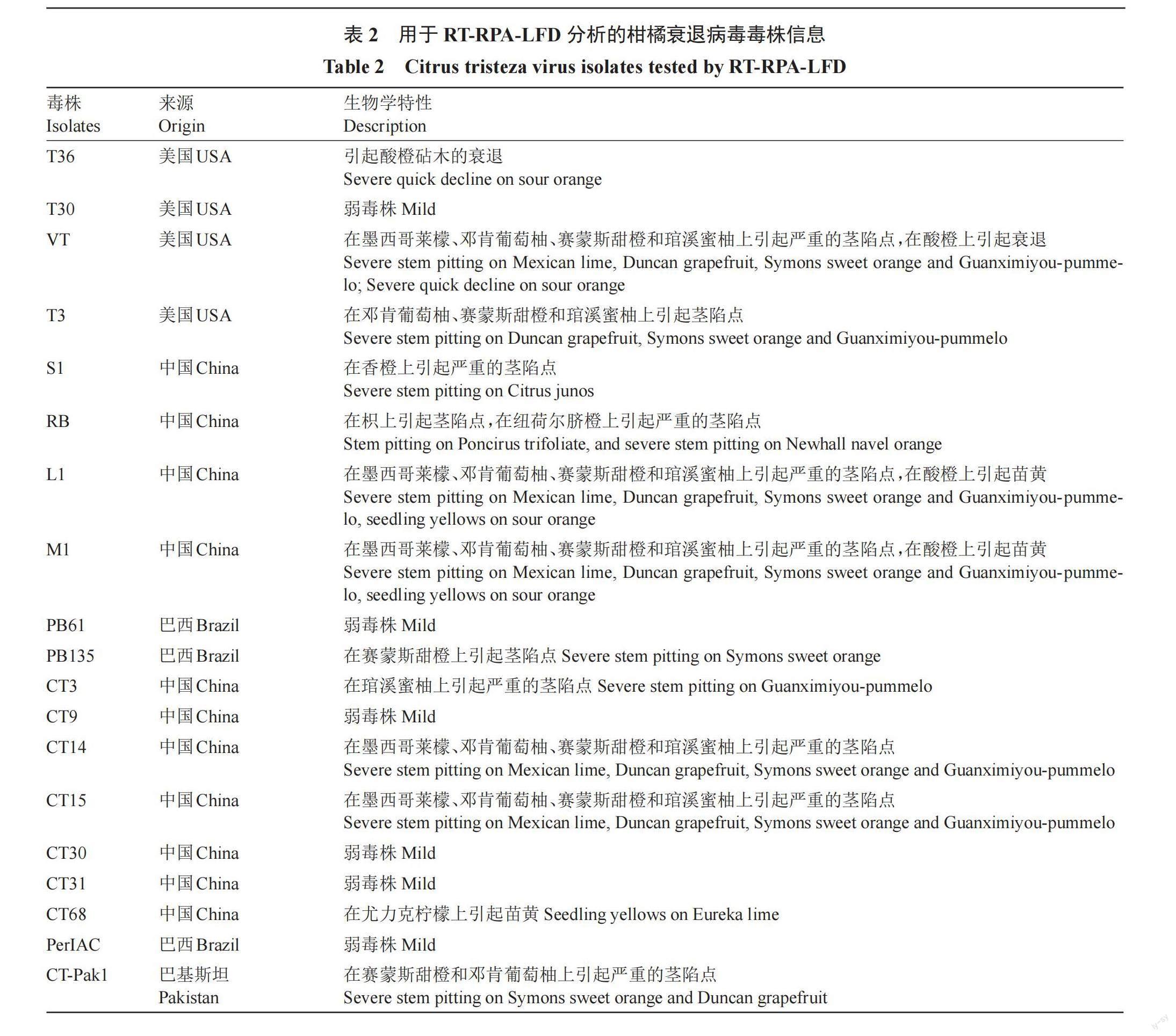
以單一感染CTV、CYVCV、CTLV、CEVd、CPV和CCDaV陽性樣品,以及無病毒柑橘樣品的核酸為模板,使用建立的RT-RPA-LFD體系進行檢測,評價其特異性。此外,為了驗證該方法是否可檢測不同基因型或來源的CTV毒株,按上述方法對T36、T30、VT、T3(由美國佛羅里達大學柑橘研究與教育中心William Dawson教授贈送)、S1、RB、L1、M1基因型毒株,以及來自澳大利亞的PB61,PB135(由巴西Centrode Citricultrua Sylvio Moreira 研究所Marcos A Machado博士贈送),來自巴西的PerIAC(由澳大利亞EMAI實驗室Patricia Barkley研究員贈送),來自巴基斯坦的CT-Pak1,以及來自中國不同產區的CT3、CT9、CT14、CT15、CT30、CT31和CT68毒株(表2)進行檢測。
1.7 RT-RPA-LFD檢測靈敏性分析
將CTV陽性樣品RNA按10倍梯度稀釋得到2.12×10-1~2.12×106拷貝·μL-1稀釋液作為模板,按照所建立的RT-RPA-LFD,以及Gillings等[12]和Yokomi等[13]的方法進行RT-PCR和RT-qPCR平行檢測,比較3種方法的靈敏度。15 μL PCR反應體系:cDNA模板1.0 μL,PrimeScript I step Enzyme Mix 0.5 μL,2×I Step Buffer 7.5 μL,CP1/CP3(10 μmol·L-1)0.3 μL。反應程序:45 ℃ 30 s;95 ℃ 2 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 1 min,36個循環;72 ℃ 5 min。25 μL RT-qPCR反應體系:2 × RT-PCR reaction mix for probe 12.5 μL,P25-F/P25-R(10 μmol·L-1)0.5 μL,CTV-CY5(10 μmol·L-1) 0.2 μL,RNA模板2 μL,iScript reverse transcriptase for one-step RT-PCR 0.5 μL。反應程序:55 ℃,2 min;95 ℃,5 min;95 ℃ 15 s,59 ℃ 30 s,40個循環。引物序列見1.4。
1.8 田間樣品檢測
將67份田間樣品按照1.3的方法提取總核酸后,分別采取RT-PCR和優化后的RT-RPA-LFD反應體系進行檢測,比較檢測效果。
2 結果與分析
2.1 引物篩選
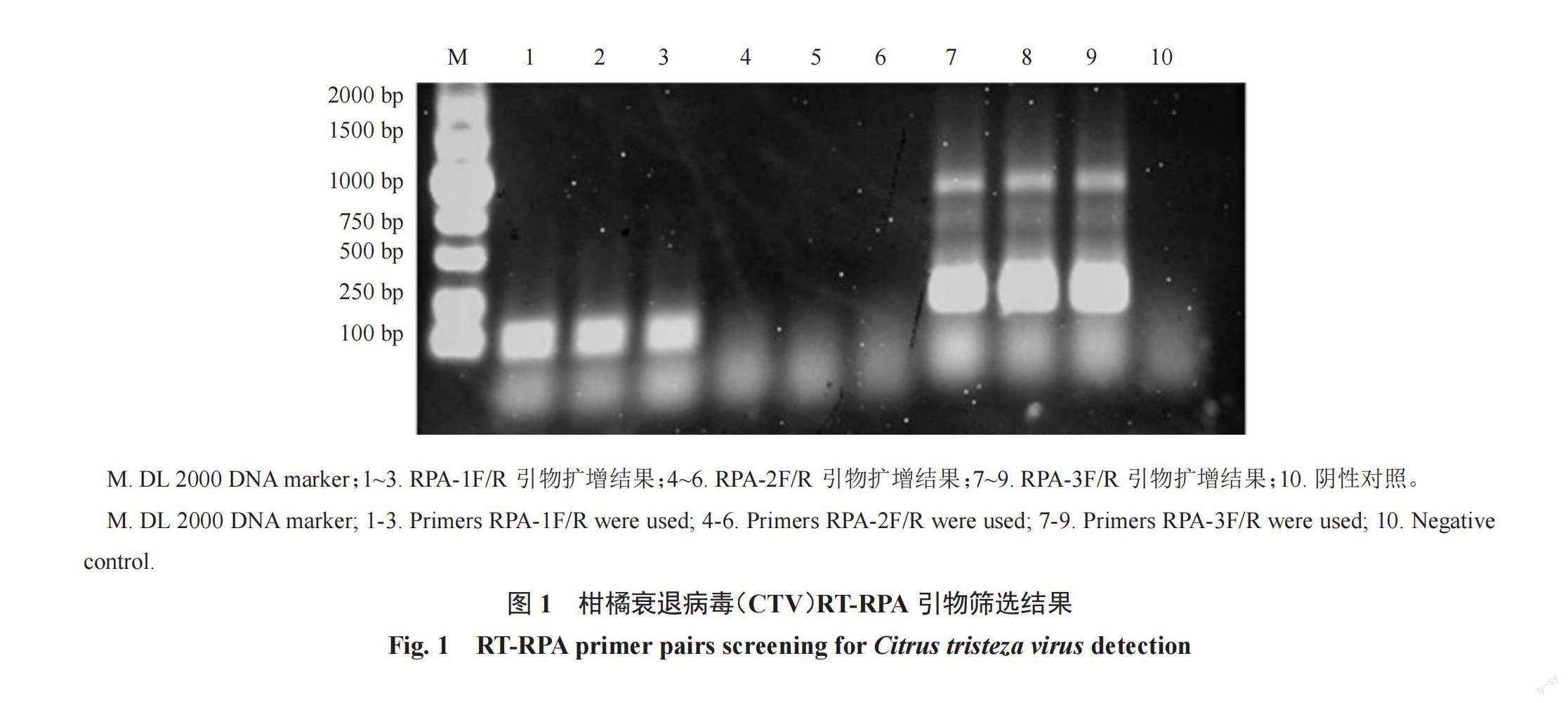
以CTV陽性樣品的總核酸為模板,分別使用設計的3對引物進行擴增。結果顯示,RPA-1F/R擴增條帶單一、明亮。所擴增產物與CTV毒株CT11A(JQ911664.1)相應序列的相似性為100%。引物RPA-2F/R無擴增條帶、RPA-3F/R存在非特異性擴增(圖1)。
2.2 RT-RPA-LFD引物濃度優化
當引物濃度為1~10 μmol·L-1時,測試線的顏色隨引物濃度的增加逐漸變深。當引物濃度高于10 μmol·L-1時,測試線無明顯變化(圖2)。因此選擇10 μmol·L-1作為RT-RPA-LFD反應最適的引物濃度。
2.3 反應時間及溫度優化
檢測結果表明,在推薦溫度37 ℃下反應超過20 min后,試紙條均出現清晰的測試線,且反應超過25 min后,測試線顏色不再加深(圖3-A)。反應時間為25 min,反應溫度10~40 ℃時,測試線顏色逐漸加深;40~45 ℃時其顏色無明顯變化,溫度高于50 ℃時,測試線不清晰(圖3-B)。綜上,確定引物濃度10 μmol·L-1,反應時間25 min,反應溫度40 ℃為最佳反應條件。
2.4 RT-RT-RPA-LFD特異性檢測
利用優化后的反應體系檢測分別感染了CTV、CYVCV、CTLV、CEV、CPV、CCDaV的樣品,以及無病毒柑橘樣品。結果顯示,僅感染CTV樣品的檢測結果呈陽性,其余樣品的檢測結果均為陰性,且能檢測出來自不同國家的19個CTV毒株。表明該反應體系與其他主要柑橘病毒無交叉反應,特異性強,且適用于對不同基因型或來源CTV毒株的檢測。
2.5 RT-RPA-LFD靈敏性檢測
將已知濃度的CTV陽性樣品按10倍梯度稀釋得到2.12×10-1~2.12×106拷貝·μL-1的稀釋液作為模板,進行RT-PCR、RT-qPCR和RT-RPA-LFD檢測(圖4)。結果表明,RT-qPCR和RT-RPA-LFD均能檢測出2.12×101拷貝·μL-1稀釋液中的CTV,而RT-PCR僅檢測出2.12×103拷貝·μL-1稀釋液中的CTV。以上結果表明RT-RPA-LFD檢測法與RT-qPCR相當,且比RT-PCR的靈敏度提高了100倍。
2.6 RT-RPA-LFD田間樣品檢測
田間樣品的檢測結果顯示,對隨機選取的67份田間樣品進行RT-PCR和RT-RPA-LFD檢測,其結果一致,均能從沃柑、紅美人、W·默科特等雜柑品種中檢測出41份CTV陽性樣品,檢出率為61.19%,經進一步驗證,其中包括T36、T30、VT、T3、T68、RB等多種基因型毒株,表明建立的RT-RPA-LFD檢測方法穩定可靠(表3,圖5)。
3 討 論
近年來,隨著我國柑橘產業的迅猛發展,柑橘衰退病隨苗木和蚜蟲傳播的速度加快,造成其在我國的發生區域不斷擴大,損失加劇[8-10]。因此準確、靈敏、便捷的病害檢測技術對于監測和防治柑橘衰退病具有重要意義。目前,CTV檢測中常用的血清學方法在檢測柚類等柑橘類型時檢出率較低[20],且基于RT-PCR的檢測方法往往依賴于多種特殊儀器設備,檢測過程復雜,專業性強。此外,雖然環介導等溫擴增技術(LAMP)靈敏度高,且操作較為簡便,但其引物設計復雜,且容易出現假陽性[21]。
筆者在本研究中根據CTV CP基因的保守區域設計引物和探針,并通過優化引物濃度、反應溫度和時間,建立、優化了CTV的RT-RPA-LFD檢測方法,其操作簡便、特異性強。與常規RT-PCR法相比,靈敏度提高了100倍,與RT-qPCR法相當。在檢測田間樣品時,RT-RPA-LFD檢測方法與常規RT-PCR法的檢測結果相同,反應時間縮短了1 h,且不需要PCR儀、凝膠成像儀等復雜儀器。由于檢測通過試紙條呈現,因此更加直觀、簡捷,可以快速準確地檢測田間樣品,有助于及時清除病株,從而最大限度地降低CTV傳播擴散的風險。此外,因為RT-RPA-LFD反應在單一管中進行,部分反應組分以凍干粉的形式保存,使得檢測過程不易發生污染。筆者在本研究中針對目前我國柑橘產業中較被追捧的多個雜柑品種進行檢測時發現,沃柑、紅美人、W·默科特等品種中CTV的檢出率較高,因此今后在引種上述品種時需要加大CTV的檢測力度。雖然Crannell等[22]的報道僅靠體溫就能完成RT-RPA-LFD反應,但在本研究中其反應仍受溫度限制,今后可進一步優化反應體系,降低反應溫度,實現在常溫下進行檢測。
4 結 論
CTV RT-RPA-LFD法特異性強、操作簡便、靈敏度高,適用于低濃度樣品檢測,且部分反應組分以凍干粉的形式保存,不易發生污染。此外,該檢測方法較RT-PCR法反應時間縮短了1 h,且不需要PCR儀、凝膠成像儀等復雜儀器,因此尤其適用于基層植保人員開展田間大規模CTV檢測。
參考文獻 References:
[1] ROISTACHER C N,MORENO P. The worldwide threat from destructive isolates of citrus tristeza virus-A review[C]//International Organization of Citrus Virologists (IOCV). International Organization of Citrus Virologists Conference Proceedings (1957-2010). California:University of California-Riverside,1991:7-19.
[2] FOLIMONOVA S Y,FOLIMONOV A S,TATINENI S,DAWSON W O. Citrus tristeza virus:survival at the edge of the movement continuum[J]. Journal of Virology,2008,82(13):6546-6556.
[3] BAR-JOSEPH M,MARCUS R,LEE R F. The continuous challenge of Citrus tristeza virus control[J]. Annual Review of Phytopathology,1989,27:291-316.
[4] HARPER S J. Citrus tristeza virus:Evolution of complex and varied genotypic groups[J]. Frontiers in Microbiology,2013,4:93.
[5] WANG J,ZHOU T Y,SHEN P,ZHANG S,CAO M J,ZHOU Y,LI Z A. Complete genome sequences of two novel genotypes of Citrus tristeza virus infecting Poncirus trifoliata in China[J]. Journal of Plant Pathology,2020,102(3):903-907.
[6] ZHOU C Y,ZHAO X Y,JIANG Y H. Boat-shaped leaf symptoms of Satsuma mandarin associated with Citrus tristeza virus (CTV) [C]//International Organization of Citrus Virologists (IOCV). International Organization of Citrus Virologists Conference Proceedings (1957-2010). California:University of California-Riverside,1996:154-157.
[7] 周彥,周常勇,李中安,王雪峰,劉科宏. 利用弱毒株交叉保護技術防治甜橙莖陷點型衰退病[J]. 中國農業科學,2008,41(12):4085-4091.
ZHOU Yan,ZHOU Changyong,LI Zhongan,WANG Xuefeng,LIU Kehong. Mild strains cross protection against stem-pitting tristeza of sweet orange[J]. Scientia Agricultura Sinica,2008,41(12):4085-4091.
[8] XIAO C,YAO R X,LI F,DAI S M,LICCIARDELLO G,CATARA A,GENTILE A,DENG Z N. Population structure and diversity of citrus tristeza virus (CTV) isolates in Hunan Province,China[J]. Archives of Virology,2017,162(2):409-423.
[9] 易龍,賴曉樺,盧占軍,鐘八蓮. 江西柑橘主產區柑橘衰退病毒分離株組群分析[J]. 植物保護,2012,38(4):112-114.
YI Long,LAI Xiaohua,LU Zhanjun,ZHONG Balian. Analysis of CP/HinfⅠRFLP groups of Citrus tristeza virus isolates in Jiangxi[J]. Plant Protection,2012,38(4):112-114.
[10] QIN Y Y,LIU Y J,ZHAO J F,HAJERI S,WANG J J,YE X,ZHOU Y. Molecular and biological characterization of a novel Citrus tristeza virus isolate that causes severe symptoms in Citrus junos cv. Ziyangxiangcheng[J]. Archives of Virology,2023,168(2):59.
[11] GARNSEY S M,PERMAR T A,CAMBRA M,HENDERSON C T. Direct tissue blot immunoassay (DTBIA) for detection of Citrus tristeza virus (CTV)[C]//International Organization of Citrus Virologists (IOCV). International Organization of Citrus Virologists Conference Proceedings (1957-2010). California:University of California-Riverside,1993:39-50.
[12] GILLINGS M,BROADBENT P,INDSTO J,LEE R. Characterisation of isolates and strains of citrus tristeza closterovirus using restriction analysis of the coat protein gene amplified by the polymerase chain reaction[J]. Journal of Virological Methods,1993,44(2/3):305-317.
[13] YOKOMI R K,SAPONARI M,SIEBURTH P J. Rapid differentiation and identification of potential severe strains of Citrus tristeza virus by real-time reverse transcription-polymerase chain reaction assays[J]. Phytopathology,2010,100(4):319-327.
[14] SAPONARI M,MANJUNATH K,YOKOMI R K. Quantitative detection of Citrus tristeza virus in Citrus and aphids by real-time reverse transcription-PCR (TaqMan?)[J]. Journal of Virological Methods,2008,147(1):43-53.
[15] BABU B,OCHOA-CORONA F M,PARET M L. Recombinase polymerase amplification applied to plant virus detection and potential implications[J]. Analytical Biochemistry,2018,546:72-77.
[16] ZENG T,CHEN X L,LIAO P,GAO H X,ZHENG C R,HUANGFU M Y,ZHOU Y. Development of transcription recombinase polymerase based isothermal amplification coupled with lateral flow immunochromatographic assay for visual detection of citrus tatter leaf virus[J]. Journal of Virological Methods,2022,309:114593.
[17] 陳玲,段續偉,張開春,張曉明,王晶,閆國華,周宇. 基于重組酶聚合酶擴增(RPA)技術的櫻桃病毒A(CVA)的檢測方法[J]. 園藝學報,2020,47(2):390-398.
CHEN Ling,DUAN Xuwei,ZHANG Kaichun,ZHANG Xiaoming,WANG Jing,YAN Guohua,ZHOU Yu. A method for the detection of cherry virus A (CVA) based on recombinase polymerase amplification (RPA) technique[J]. Acta Horticulturae Sinica,2020,47(2):390-398.
[18] 陳玲,閆國華,張曉明,周宇,王晶,段續偉,李彥林,張開春. 李矮縮病毒重組酶聚合酶擴增—側流層析試紙條檢測方法的建立[J]. 園藝學報,2021,48(1):183-192.
CHEN Ling,YAN Guohua,ZHANG Xiaoming,ZHOU Yu,WANG Jing,DUAN Xuwei,LI Yanlin,ZHANG Kaichun. Establishment of recombinase polymerase amplification combined with lateral flow dipstick for detection of prune dwarf virus[J]. Acta Horticulturae Sinica,2021,48(1):183-192.
[19] ZHANG S L,RAVELONANDRO M,RUSSELL P,MCOWEN N,BRIARD P,BOHANNON S,VRIENT A. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP? using reverse transcription-recombinase polymerase amplification[J]. Journal of Virological Methods,2014,207:114-120.
[20] 劉科宏,周彥,王雪峰,唐科志,周常勇. 柑橘衰退病毒在3種寄主不同組織中分布的研究初報[J]. 西北農林科技大學學報(自然科學版),2005,33(S1):109-110.
LIU Kehong,ZHOU Yan,WANG Xuefeng,TANG Kezhi,ZHOU Changyong. Distribution of Citrus tristeza virus among three hosts in different tissues[J]. Journal of Northwest A & F University (Natural Science Edition),2005,33(S1):109-110.
[21] WARGHANE A,MISRA P,BHOSE S,BISWAS K K,SHARMA A K,REDDY M K,GHOSH D K. Development of a simple and rapid reverse transcription-loop mediated isothermal amplification (RT-LAMP) assay for sensitive detection of Citrus tristeza virus[J]. Journal of Virological Methods,2017,250:6-10.
[22] CRANNELL Z A,ROHRMAN B,RICHARDS-KORTUM R. Equipment-free incubation of recombinase polymerase amplification reactions using body heat[J]. PLoS One,2014,9(11):e112146.

