COP9亞基FaCSN5在草莓果實發育過程中的功能分析
高佳慧 冀桂明 李文靜 郭家選 沈元月 高凡
摘? ? 要:【目的】探究草莓COP9信號復合體亞單位5(constitutive photomorphogenic signalosome subunit 5,CSN5)在草莓果實發育過程中的功能。【方法】以草莓品種紅顏為材料,根據草莓果實發育過程中的轉錄組數據,篩選并克隆FaCSN5基因。基于生物信息學對其功能域、理化性質、蛋白結構等進行預測。通過SDS-PAGE和Western Blot檢測FaCSN5-His目的蛋白,利用煙草對其進行亞細胞定位分析。利用RT-qPCR檢測FaCSN5的時空表達水平,利用農桿菌介導瞬時侵染草莓果實,觀察記錄表型,檢測FaCSN5基因表達水平。利用圓片溫育和外源激素處理試驗檢測外源激素對FaCSN5基因表達的誘導影響。【結果】系統進化樹分析表明FaCSN5、FvCSN5和月季CSN5b同源性較高,相似率分別為100%和94.24%。克隆的FaCSN5與艷麗草莓8條基因序列的堿基和氨基酸序列相似率分別達98.97%和99.35%。FaCSN5基因的編碼區為1080 bp,編碼359個氨基酸,具有一個保守的MPN結構域。pET30a-FaCSN5融合表達載體的大腸桿菌原核表達表明FaCSN5-His目的蛋白大小約66 ku。亞細胞定位顯示FaCSN5-GFP融合蛋白定位為細胞核和細胞質。FaCSN5在根中表達水平最高,在種子中最低,在根中的表達量是種子中表達量的8倍;果實發育過程中在全紅期表達量最高,在褪綠期表達量最低,從褪綠期開始隨著果實發育表達量升高。農桿菌介導瞬時侵染草莓果實,FaCSN5過表達能夠促進草莓果實成熟;沉默FaCSN5表達則會抑制草莓果實成熟。FaCSN5啟動子上含有響應茉莉酸甲酯和赤霉素的順式作用元件,且其表達受到這兩種激素及脫落酸的誘導。【結論】FaCSN5可能是通過多種激素調控促進草莓果實成熟。
關鍵詞:草莓;紅顏;CSN5;表達分析;亞細胞定位;激素誘導
中圖分類號:S668.4 文獻標志碼:A 文章編號:1009-9980(2023)12-2536-12
收稿日期:2023-09-11 接受日期:2023-10-22
基金項目:國家自然科學基金資助項目(32272648,32072516,32030100)
作者簡介:高佳慧,女,在讀碩士研究生,研究方向為果實發育與品質調控。E-mail:gjh696969@126.com
*通信作者Author for correspondence. E-mail:gaofan@bua.edu.cn
Functional analysis of the COP9 subunit FaCSN5 during strawberry fruit development
GAO Jiahui1, JI Guiming2, LI Wenjing2, GUO Jiaxuan2, SHEN Yuanyue1, GAO Fan2*
(1College of Plant Science and Technology, Beijing University of Agriculture/Beijing Key Laboratory of New Technology in Agricultural Application, Beijing 102206, China; 2College of Bioscience and Resources Environment, Beijing University of Agriculture/Key Laboratory for Northern Urban Agriculture of Ministry of Agriculture and Rural Affairs, Beijing 102206, China)
Abstract: 【Objective】 The study aimed to investigate the function of strawberry (Fragaria × ananassa) CSN5 (constitutive photomorphogenic signalosome subunit 5) during strawberry fruit development. 【Methods】 Benihoppe strawberry was used as experimental material. Firstly, based on the transcriptome data of the five developmental stages (SG, LG, Wt, IR and PR) of strawberry fruit, a gene with increased expression level during fruit development from the large green fruit was screened. Because it contained MPN conserved domain and had 100% sequence similarity with diploid strawberry FvCSN5 (XM_004291211), the gene was named FaCSN5. Total RNA was extracted from the samples using a total RNA extraction kit (Guangzhou Magen Biotechnology Co., Ltd.), and the cDNA was synthesized by reverse transcription using Hi fair ? Ⅲ 1st Strand cDNA Synthesis Super Mix for qPCR (Shanghai Yeasen Biotechnology Co., Ltd.) kit, and then the full-length CDS sequence of FaCSN5 was obtained by PCR. Secondly, some bioinformatics techniques were used in this study. The molecular formula, molecular weight, isoelectric point, and fat solubility index of encoding protein were analyzed in the Prot Param website. The conserved domain of FaCSN5 was analyzed by the NCBI website and Blast tool. The transmembrane domain of FaCSN5 protein was analyzed by TMHMM 2.0. The secondary and tertiary structures of FaCSN5 were analyzed online using NPS and Swiss Model. The phylogenetic tree of FaCSN5 homologous genes was constructed by MEGA11 software. Thirdly, the spatiotemporal expression levels of FaCSN5 were detected by RT-qPCR using Agrobacterium-mediated transient transformation of strawberry fruits, and the phenotypes were observed and used to detect the expression levels of the FaCSN5 gene. The cis-acting elements of FaCSN5 gene promoter were analyzed by the online software Plant CARE. The induction of FaCSN5 gene expression by exogenous hormones was detected by disc incubation and exogenous hormone treatment experiments. The subcellular localization was observed by Agrobacterium-mediated transient transformation of tobacco mesophyll cells. Finally, the full-length CDS sequence of FaCSN5 was constructed into pET30a vector by homologous recombination, and the pET30a-FaCSN5 fusion expression vector was obtained induced and purified. Western Blot was used to detect FaCSN5-His target protein by SDS-PAGE and Anti-His antibody. 【Results】 Evolutionary tree analysis showed that FaCSN5 was highly homologous to FvCSN5 and rosa CSN5b, with similarity rates of 100% and 94.24%, respectively. The base and amino acid sequence similarity between the cloned FaCSN5 and the eight gene sequences of Yanli strawberry reached 98.97% and 99.35%, respectively. Bioinformatics analysis showed that the coding sequence of FaCSN5 was 1080 bp, encoding 359 amino acids. The analysis of physicochemical properties of amino acids showed that the molecular formula of FaCSN5 was C1797H2773N471O558S14, the molecular weight of the protein was 40.35 ku, and the isoelectric point (pI) was 4.93, which was an acidic protein. The protein contained 48 negatively charged amino acid residues (Asp + Glu) and 31 positively charged amino acid residues (Arg + Lys). The instability coefficient was 41.41, and the average hydrophilicity is -0.421. It was inferred that the protein should be an unstable hydrophobic protein. Conserved domain analysis showed that FaCSN5 had a conserved MPN domain. Phylogenetic tree analysis showed that FaCSN5 had high homology with FvCSN5 and rose CSN5b, and the similarity rates were 100% and 94.24%, respectively. The pET30a-FaCSN5 fusion expression vector was constructed for prokaryotic expression in Escherichia coli. The results of SDS-PAGE and Western Blot showed that the size of FaCSN5-His target protein was about 66 ku. The transient expression of Nicotiana benthamiana showed that the FaCSN5-GFP fusion protein was localized in the nucleus and cytoplasm. RT-qPCR analysis showed that the expression level of FaCSN5 was the highest in the root, followed by FR, PR, stem, leaf, and flower. The expression level of FaCSN5 was the lowest in the seed, and the expression level in the root was 8 times higher than that in the seed, indicating that FaCSN5 had tissue specificity. During fruit development, the expression level was the highest at the Full red stage and the lowest at the De-greening stage. From the Degreening green stage, the expression level increased with fruit development, indicating that FaCSN5 might be involved in the development of strawberry fruit. Agrobacterium-mediated transient infestation of strawberry fruit with FaCSN5 overexpression could promote strawberry fruit ripening; silencing FaCSN5 expression inhibited strawberry fruit ripening. The FaCSN5 promoter contained cis-acting elements in response to methyl jasmonate and gibberellin. After treatment with MeJA and GA, the expression level of FaCSN5 gene was slightly lower than that of the control group after 1 h of MeJA treatment. After 2-5 h of treatment, the expression level of FaCSN5 gene was higher than that of the control group. The expression level of FaCSN5 gene was higher than that of the control group at 1-5 h after GA treatment, and the expression level was the highest at 1 h after treatment. After ABA treatment, the expression level of FaCSN5 gene was higher than that of the control group, and the expression level was the highest after 3 h of treatment. The results showed that the expression level of FaCSN5 gene was induced by MeJA, GA, and ABA, to various degrees. 【Conclusion】 The protein height of FaCSN5 was about 66 ku, which was localized in the nucleus and cytoplasm. The expression of FaCSN5 was induced by abscisic acid, methyl jasmonate, and gibberellin. FaCSN5 might promote strawberry fruit ripening through multiple hormonal regulations.
Key words: Strawberry; Benihoppe; CSN5; Expression analysis; Subcellular localization; Hormone induction
COP9信號復合體(constitutively photomorphogennic signalosome,CSN)最初從擬南芥中被鑒定為光形態發生的重要調節體[1]。CSN是泛素-蛋白酶體途徑中一種進化上高度保守的多蛋白復合物,其特異性地將泛素化的蛋白質引導至26S蛋白酶體,從而促進植物降解[2]。在高等真核生物中,CSN亞基命名法已經統一,各個CSN亞基現在被稱為CSN1-CSN8[3]。CSN亞基可以獨立地行使功能,也可以結合成CSN復合體發揮作用,其中CSN5的作用最受關注[4]。在擬南芥中發現AtCSN5a和AtCSN5b基因,均為編碼CSN5的基因[5]。在草莓、葡萄、水稻的基因組中,則只含有一個CSN5基因[6-7]。
CSN5包含MPN(Mpr1p-Pad1p-N-terminal)結構域,主要識別信號因子,激活下游信號,從而調控一系列生物學功能,MPN域蛋白具有生化活性,完整的CSN5是COP9信號復合體穩定所必需的[8]。目前CSN5已經在多個物種中被克隆并分析,各種功能也陸續被發現,如雙子病毒C2蛋白與CSN5相互作用并改變擬南芥中基于CUL1的SCF泛素E3連接酶,最終涉及激素信號調控[9]。在番茄中CSN4和CSN5通過JA信號通路調節COI1和響應根結線蟲感染[10]。CSN5a通過熱脅迫來恢復AUX/IAA水平,從而調節擬南芥中生長素含量[11]。因此,CSN5在生物脅迫和非生物脅迫中起著重要的調控作用。然而,CSN5在草莓上的分子基礎目前還不清楚。
草莓是研究非呼吸躍變型果實發育成熟機制的模式植物[12-13]。基于筆者實驗室草莓果實中的5個發育時期的轉錄組分析,篩選到一個隨著果實發育表達量增加的COP9亞基,編碼MPN保守結構域,將其命名為FaCSN5,并推測其可能涉及草莓果實發育調控。筆者在本研究中首先從八倍體草莓紅顏果實中克隆到FaCSN5的CDS序列,并對其理化性質、蛋白結構、蛋白純化、亞細胞定位、組織表達和農桿菌瞬時侵染草莓果實等進行預測和研究,為深入研究FaCSN5在草莓果實發育中的分子機制和功能奠定基礎。
1 材料和方法
1.1 材料
試驗材料為北京農學院科技園區日光溫室基質栽培的八倍體草莓紅顏(Fragaria × ananassa ‘Benihoppe)。溫室內晝夜氣溫范圍為10~30 ℃、空氣相對濕度為60%~80%、日照時間為8 h,肥水按當地常規管理。在2023年2月第二茬果時,于上午10:00對根、莖、葉、花、果實和種子各組織部位取樣。根據前人的研究[14],采集小綠期、大綠期、褪綠期、白果期、始紅期、片紅期和全紅期等不同發育時期的草莓果實,并用液氮速凍,-80 ℃保存。
1.2 總RNA提取及反轉錄
采用難提植物總RNA小提試劑盒(美基生物科技有限公司)提取樣品總RNA,利用Hi fair? Ⅲ 1st Strand cDNA Synthesis Super Mix for qPCR(翌圣生物公司)試劑盒反轉錄合成cDNA,放置-80 ℃保存備用。
1.3 草莓CSN5生物學分析
FaCSN5保守結構域通過NCBI(https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)預測;FaCSN5蛋白的跨膜結構域使用TMHMM 2.0(https://services.healthtech.dtu.dk/services/TMHMM-2.0/)分析;FaCSN5分子質量、等電點、蛋白質分子式和不穩定系數利用Prot Param(https://web.expasy.org/protparam/)在線預測;FaCSN5蛋白質二級和三級結構利用NPS(https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_gor4.html)和Swiss Model(http://swissmodel.expasy.org/)在線分析;根據得到的FaCSN5氨基酸序列,利用MEGA軟件進行氨基酸序列的同源性比對和系統進化樹構建;通過在線軟件Plant CARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)分析FaCSN5啟動子的順式作用元件。
1.4 圓片溫育與激素處理
取長勢一致的白果期果實,使用打孔器和刀片將果實制備成圓片,放于溫育平衡液中平衡30 min,其圓片直徑和厚度分別為0.75 cm和0.1 cm,溫育平衡液包含10 mmol·L-1 MgCl2、5 mmol·L-1 CaCl2、10 mmol·L-1 EDTA、5 mmol·L-1維生素C、200 mmol·L-1甘露醇和50 mmol·L-1 MES(pH = 5.5)。將平衡好的果實圓片隨機取5 g分別放入到含有100 μmol·L-1茉莉酸甲酯(methyl jasmonate,MeJA)、100 μmol·L-1赤霉素(gibberellin,GA)和100 μmol·L-1脫落酸(abscisic acid,ABA)的平衡液中,25 ℃、140 r·min-1孵育。于1、2、3、4、5 h分別取1 g處理好的圓片,液氮速凍,-80 ℃保存。
1.5 實時熒光定量PCR
利用Primer 5軟件設計實時熒光定量引物(表1),以紅顏草莓根、莖、葉、花、種子、各發育時期的果實和圓片溫育樣品的cDNA為模板,草莓的Actin基因為內參基因,采用Bio-Rad CFX96熒光定量PCR儀檢測FaCSN5在不同部位和不同處理下的表達情況。擴增程序使用三步法反應程序,實時熒光定量加樣體系和反應程序均參考Trans Start Top Green qPCR Super Mix(全式金生物技術有限公司)說明書。試驗采取3次重復,相對表達量采用2-ΔΔCt計算。
1.6 草莓FaCSN5原核表達載體構建及蛋白純化
基于二倍體森林草莓同源基因FvCSN5(XM_004291211),采用Snap Gene軟件設計引物(表1),以紅顏草莓的cDNA為模板,選用2 × Phanta Max Master Mix(諾唯贊生物科技股份有限公司)克隆攜有pET30a載體同源臂的目的片段,PCR產物經1%瓊脂糖凝膠電泳檢測后,用瓊脂糖凝膠DNA回收試劑盒(美基生物科技有限公司)回收目的條帶。使用內切酶EcoRⅠ和SalⅠ對pET30a表達載體進行雙酶切,通過1%的瓊脂糖凝膠電泳進行分離,將pET30a載體片段切膠回收。選用同源重組酶Clon Express Ⅱ One Step Cloning Kit(諾唯贊生物科技有限公司)連接攜有載體同源臂的FaCSN5目的片段和pET30a載體片段。將連接產物轉化到大腸桿菌DH5α(唯地生物技術有限公司),菌落PCR驗證后將陽性菌送金唯智生物科技有限公司進行測序。
提取重組質粒,命名為pET30a-FaCSN5,轉化大腸桿菌感受態BL21(DE3),涂板于含有卡那霉素(100 mg·L-1)LB固體培養基上37 ℃過夜培養,菌落PCR驗證。挑取陽性單菌落,接種于20 mL含有卡那霉素(100 mg·L-1)LB液體培養基上,在37 ℃、220 r·min-1活化4~6 h,將菌液加至300 mL含有卡那霉素(100 mg·L-1)的LB液體培養基中,調OD600值為0.1,在搖床上繼續搖動,直到OD600值為0.6~0.8,加入終濃度為0.5 mmol·L-1的IPTG,在16 ℃、140 r·min-1誘導過夜。收集誘導后菌液300 mL,4 ℃離心機8000 r·min-1離心4 min,向沉淀中加入30 mL Binding Buffer(PBS緩沖液,5 mmol·L-1咪唑,調pH至7.4)和0.3 mL 100 ?mol·L-1 PMSF,超聲20 min(350 W,超聲開3 s,超聲關2 s),4 ℃離心機8000 r·min-1離心15 min,取上清液。利用Ni-NTA純化目標蛋白,具體操作參照其說明書,采用含有500 mmol·L-1咪唑的PBS洗脫蛋白,參考楊潔[15]的方法進行SDS-PAGE和Western Blot檢測。
1.7 亞細胞定位
選定酶切位點SpeⅠ和KpnⅠ對pCAMBIA-Super1300-GFP表達載體進行雙酶切,利用Snap Gene軟件設計引物(表1),使用攜有載體同源臂的引物克隆目的片段,采用同源重組法連接載體片段和目的片段。經菌落PCR驗證的陽性菌,送金唯智生物科技有限公司測序驗證,提取重組質粒35S::FaCSN5-GFP,過表達載體也用該重組質粒,將其轉化農桿菌GV3101,對煙草下表皮進行侵染,3 d后在激光共聚焦下觀察pCAMBIA-Super1300-GFP和35S::FaCSN5-GFP的綠色熒光在煙草細胞的位置。
1.8 FaCSN5 RNAi載體構建
Gateway的方法構建FaCSN5 RNAi載體,設計引物(表1),BP反應具體操作見賽默飛Gateway? BP Clonase? Ⅱ酶混合物說明書,LR反應具體操作見賽默飛Gateway? LR Clonase? Ⅱ酶混合物說明書。
1.9 FaCSN5瞬時侵染草莓果實
對FaCSN5 RNAi、轉化的空載體(對照)和OE陽性農桿菌,挑取陽性單菌落于5 mL含有50 ?g·mL-1壯觀霉素或50 ?g·mL-1卡那霉素和50 ?g·mL-1利福平的YEB液體培養基中,28 ℃,200 r·min-1水平震蕩培養過夜。取適量菌液加入到10 mL含有50 ?g·mL-1卡那霉素和50 ?g·mL-1利福平的YEB液體培養基中,調OD600值為0.1,28 ℃,200 r·min-1震蕩培養至OD600值為1.0~1.2。6000 r·min-1離心5 min,收集菌體,用果實侵染液重懸菌體,調OD600值為0.4~0.6,避光、28 ℃靜置2 h,果實侵染液包含10 mmol·L-1 MES、10 mmol·L-1 MgCl2和200 ?mol·L-1 AS。挑選發育健康的褪綠期果實做上標記,每種農桿菌各注射6個,每天拍照記錄表型,注射6 d后取樣,去掉種子切取注射部位,液氮速凍,-80 ℃保存。試驗設置3次重復。
2 結果與分析
2.1 草莓FaCSN5基因的克隆
紅顏草莓cDNA為模板的PCR擴增條帶大小約1080 bp(圖1-A),在小綠期(SG)、大綠期(LG)、白果期(Wt)、始紅期(IR)、片紅期(PR)5個草莓果實發育時期,COP9亞基的表達量從大綠期開始隨著果實發育而增加(圖1-B)。采用MEGA11鄰接法(Neighbor-Joining method)構建的系統發育樹顯示該基因的編碼區(coding sequence,CDS)也為1080 bp,與二倍體草莓FvCSN5(XM_004291211)和月季CSN5b(XM_024310633.2)同源性非常高,相似率分別達100%和94.24%(圖2),因此將該基因命名為FaCSN5。此外,栽培品種艷麗草莓從GDR(https://www.rosaceae.org/)Blast獲得了8條基因序列[16],克隆的FaCSN5與艷麗草莓8條基因序列的堿基和氨基酸序列相似率分別達98.97%和99.35%。
2.2 草莓FaCSN5基因的生物信息學分析
FaCSN5基因的分子式為C1797H2773N471O558S14,編碼359個氨基酸,蛋白質分子質量為40.35 ku,等電點(pI)為4.93,為酸性蛋白,含有31個正電荷氨基酸殘基(Arg + Lys),48個負電荷氨基酸殘基(Asp + Glu),不穩定系數是41.41,親水性平均值為-0.421,因此,FaCSN5蛋白可能為不穩定的疏水性蛋白。
利用NCBI網站以及Blast工具分析FaCSN5蛋白的保守結構,結果顯示FaCSN5在51~323位氨基酸之間含有一個MPN保守結構域(圖3)。采用TMHMM 2.0對蛋白的跨膜結構域進行分析,發現FaCSN5無跨膜結構(圖4)。
利用NPS和Swiss Model在線分析蛋白質二級和三級結構(圖5)。FaCSN5蛋白的二級結構由4個結構組成,分別為α螺旋(α-helix)、β轉角(Beta turn)、無規則卷曲(random coil)和延伸鏈(extended strand)(圖5-A),Alpha helix包含144個氨基酸,占比為40.11%;Beta turn包含11個氨基酸,占比為3.06%;Random coil 包含152個氨基酸,占比為42.34%;Extended strand包含52個氨基酸,占比為14.48%。
2.3 草莓FaCSN5的原核表達分析
FaCSN5-His原核表達分析如圖6所示,對包括SDS-PAGE(圖6-A)及Anti-His抗體進行Western Blot檢測(圖6-B),FaCSN5-His目的蛋白條帶大小在55~70 ku之間,約66 ku,其中含His標簽0.84 ku。
2.4 草莓FaCSN5的亞細胞定位
草莓FaCSN5-GFP融合蛋白的亞細胞定位分析如圖7所示,細胞核和細胞質有綠色熒光,表明FaCSN5-GFP融合蛋白定位于細胞核和細胞質。
2.5 草莓FaCSN5的時空表達
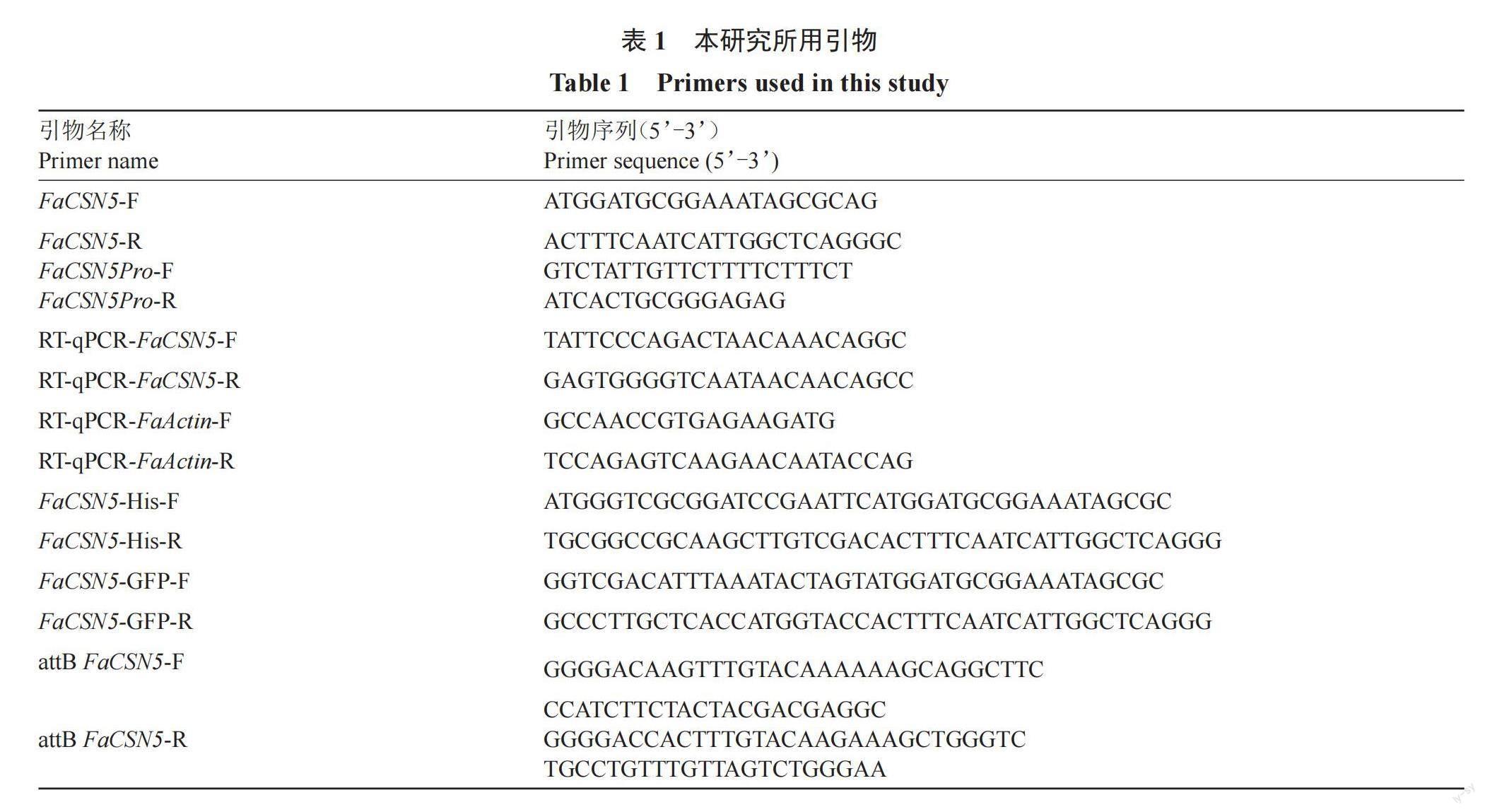
草莓根、莖、葉、花、種子和全紅期果實的FaCSN5相對表達量如圖8-A所示,根的FaCSN5相對表達量最高,其次是全紅期果實、莖、葉和花,種子的表達量最低。其中,根的FaCSN5表達量可達種子的8倍,表明FaCSN5具有明顯的組織特異性。如圖8-B所示,在草莓果實發育的不同階段,FaCSN5相對表達量從褪綠期開始就一直呈現上升的趨勢,在全紅時期達到最高,表明FaCSN5參與了草莓果實的發育。
2.6 草莓FaCSN5促進草莓果實成熟
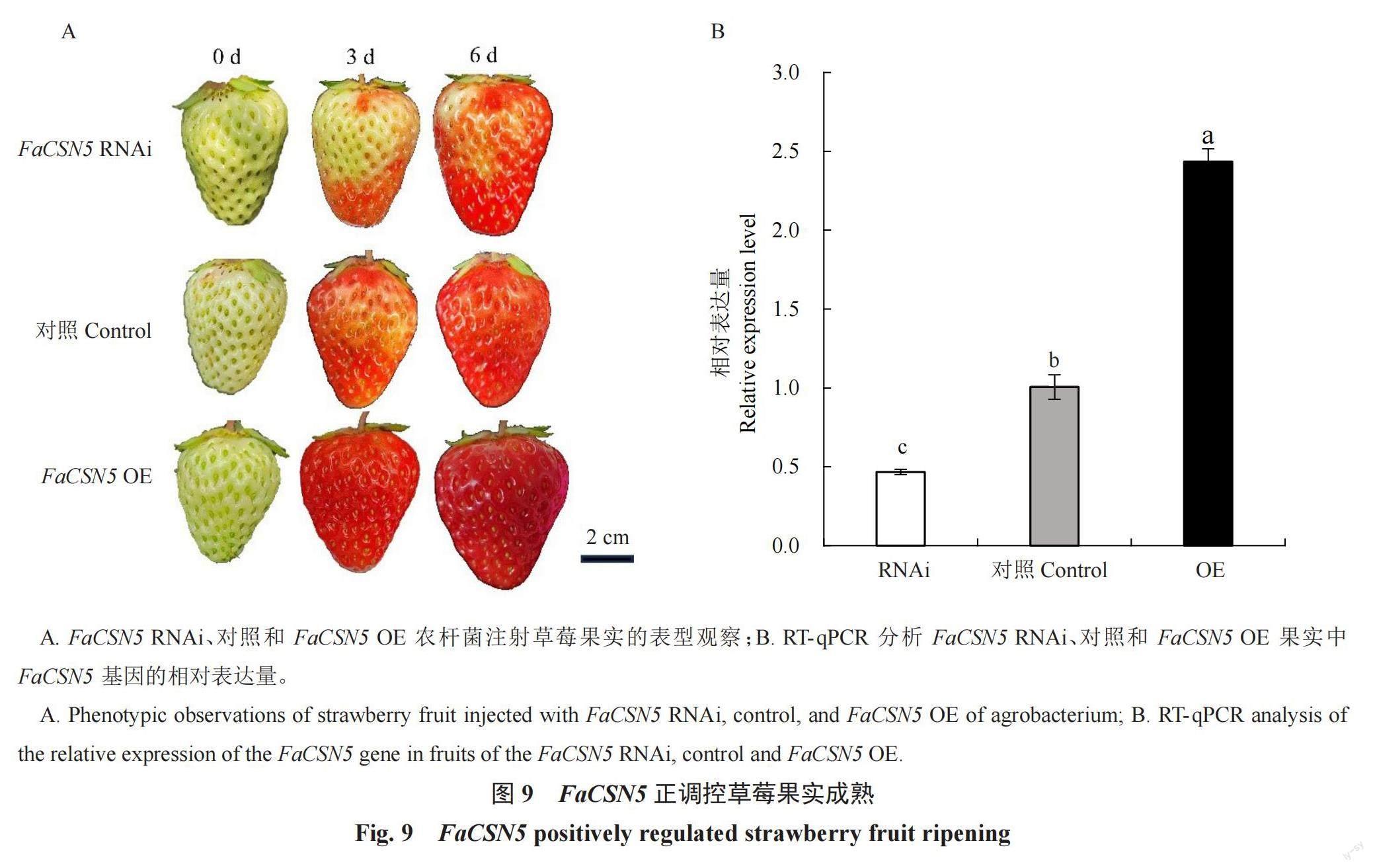
為驗證FaCSN5在草莓果實發育中的功能,分別構建FaCSN5 RNAi和FaCSN5 OE載體,轉化GV3101農桿菌,通過農桿菌介導瞬時侵染草莓果實,每天拍照記錄表型(圖9-A),通過RT-qPCR檢測FaCSN5表達水平(圖9-B)。結果表明,FaCSN5過表達能夠促進草莓果實成熟;沉默FaCSN5表達則會抑制草莓果實成熟。以上結果初步證明FaCSN5在草莓果實成熟過程中起著重要作用。
2.7 草莓FaCSN5在激素誘導下的表達分析
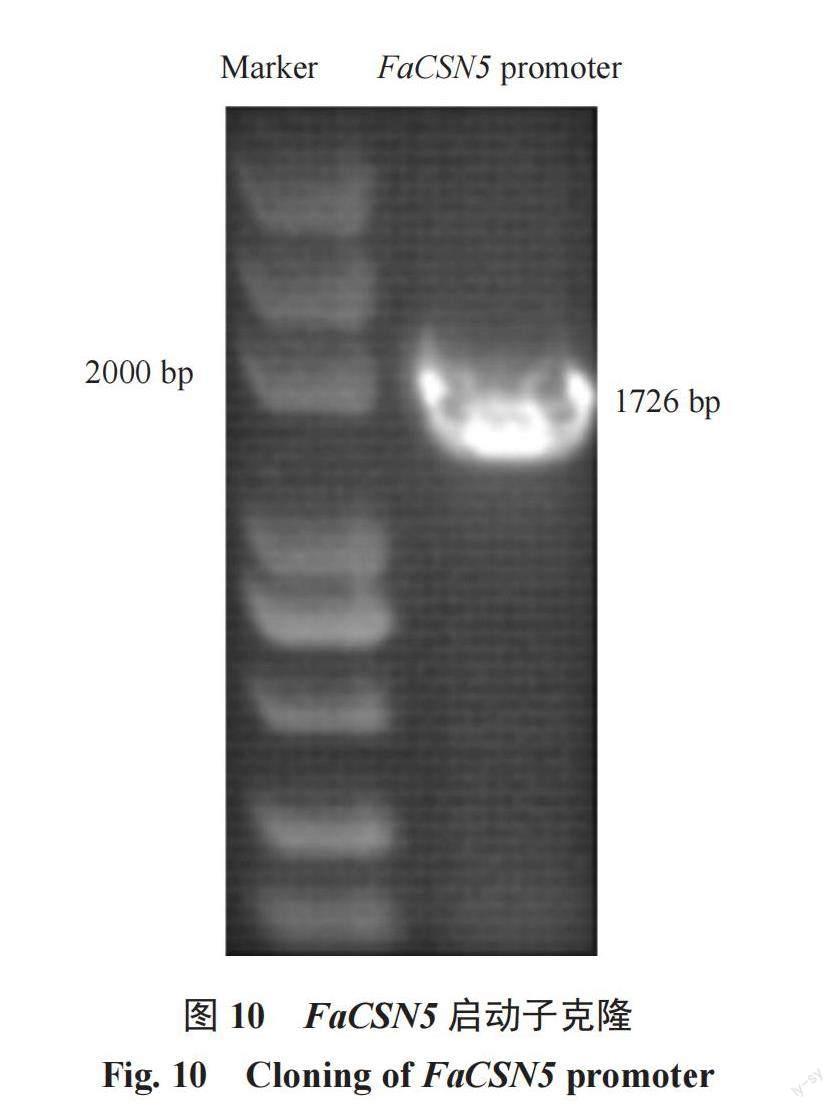
為了進一步分析FaCSN5的功能,以紅顏草莓基因組DNA為模板,擴增FaCSN5起始密碼子至上游約2000 bp的堿基序列(圖10),PCR產物長度1726 bp,與NCBI查詢到的二倍體草莓啟動子序列進行比對,其相似率為100%。將FaCSN5啟動子序列與八倍體草莓艷麗的啟動子序列[16]進行比較,發現2條啟動子序列符合條件,并且與FaCSN5啟動子序列的相似率達88.07%。表2顯示了FaCSN5基因啟動子的順式作用元件及其功能,FaCSN5啟動子有不同類型的響應元件,它們的功能也各不相同,其中,茉莉酸甲酯響應元件、赤霉素響應元件為激素響應元件。
FaCSN5基因表達水平會受到MeJA、GA和ABA不同程度的誘導,圖11表示不同外源激素處理草莓果實后FaCSN5基因表達水平。外施MeJA處理1 h后,FaCSN5基因表達水平略低于對照組,處理后的2~5 h,FaCSN5基因表達水平均高于對照組;外施GA處理1~5 h后,FaCSN5基因表達水平均高于對照組,在處理1 h后表達量最高。外施ABA處理后FaCSN5基因表達水平均高于對照組,且在處理3 h后表達量最高。
3 討 論
草莓是薔薇科多年生草本植物,味道甘甜,香氣濃郁,含有豐富的營養物質。草莓是研究非呼吸躍變型果實發育成熟機制的模式植物[12-13]。草莓果實發育和成熟受到諸多因素的影響,如溫度、光照等因素的影響,其中植物激素的協同作用扮演了重要的角色[17]。
CSN參與協調植物的生長發育[18]。筆者在本研究中以草莓果實不同發育時期轉錄組數據為基礎,克隆出草莓CSN5基因。FaCSN5只有一個MPN功能域,系統發育分析表明,其與二倍體草莓和月季等薔薇科植物的進化關系較近,說明CSN5在進化過程中結構和功能較為保守。原核表達FaCSN5-His后,通過SDS-PAGE和Western Blot檢測,發現FaCSN5-His目的蛋白條帶約66 ku。通過亞細胞定位分析,FaCSN5-GFP融合蛋白定位于細胞核和細胞質。
關于CSN的表達模式已有一些研究報道,如GmCSN5s在大豆的根和根瘤中的表達量高于其他組織[19];HbCSN5在橡膠樹的膠乳中表達量最高,在雄花中表達量最低[20];OsCSN5基因在水稻的莖尖分生組織中表達量較高[21]。筆者在本研究中利用RT-qPCR檢測了草莓根、莖、葉、花、種子和全紅期果實的相對表達量,發現FaCSN5在草莓根中表達量最高,其次是全紅期果實、莖、葉和花,種子的表達量最低,表明FaCSN5具有組織特異性。FaCSN5在全紅期表達量最高,在褪綠期表達量最低,從褪綠期到全紅期,FaCSN5表達量呈現上升的趨勢,表明FaCSN5可能參與草莓果實的發育。為了驗證FaCSN5在果實發育過程中的功能,筆者通過農桿菌介導瞬時侵染草莓果實記錄表型,檢測FaCSN5的表達水平,結果表明,FaCSN5過表達能夠促進草莓果實成熟;沉默FaCSN5表達則會抑制草莓果實成熟。以上研究表明FaCSN5在草莓果實成熟過程中起著重要作用。
CSN參與包括乙烯、脫落酸、生長素、水楊酸等多種植物激素的調控過程[22-25]。OsCSN1通過CUL1的E4連接酶降解SLR3,最終影響內源激素GA的合成[26]。筆者在本研究中發現FaCSN5啟動子區域含有茉莉酸甲酯和赤霉素激素響應元件,推測FaCSN5基因表達可能受到這兩種激素誘導。因此,通過圓片溫育和外源激素處理試驗,發現茉莉酸甲酯和赤霉素處理后,FaCSN5基因表達明顯受到誘導,且顯著響應赤霉素。草莓是非呼吸躍變型果實,脫落酸在草莓果實發育過程中起著重要的作用,通過脫落酸處理后,發現FaCSN5基因表達顯著受到脫落酸的調控。
綜上所述,FaCSN5基因可能通過多種激素信號轉導途徑促進草莓果實的成熟。
4 結 論
FaCSN5蛋白條帶約66 ku,在細胞核和細胞質存在定位。FaCSN5表達受到脫落酸、茉莉酸甲酯和赤霉素的誘導調控,推測FaCSN5基因可能通過多種激素信號轉導途徑促進草莓果實的成熟。總之,本研究結果為深入研究FaCSN5在草莓果實中的分子機制和功能奠定了基礎。
參考文獻References:
[1] WEI N,CHAMOVITZ D A,DENG X W. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development[J]. Cell,1994,78(1):117-124.
[2] SINHA A,ISRAELI R,CIRIGLIANO A,GIHAZ S,TRABELCY B,BRAUS G H,GERCHMAN Y,FISHMAN A,NEGRI R,RINALDI T,PICK E. The COP9 signalosome mediates the Spt23 regulated fatty acid desaturation and ergosterol biosynthesis[J]. The FASEB Journal,2020,34(4):4870-4889.
[3] QIN N X,XU D Q,LI J G,DENG X W. COP9 signalosome:Discovery,conservation,activity,and function[J]. Journal of Integrative Plant Biology,2020,62(1):90-103.
[4] DUBIEL D,ROCKEL B,NAUMANN M,DUBIEL W. Diversity of COP9 signalosome structures and functional consequences[J]. FEBS Letters,2015,589(19):2507-2513.
[5] KWOK S F,SOLANO R,TSUGE T,CHAMOVITZ D A,ECKER J R,MATSUI M,DENG X W. Arabidopsis homologs of a c-Jun coactivator are present both in monomeric form and in the COP9 complex,and their abundance is differentially affected by the pleiotropic cop/det/fus mutations[J]. The Plant Cell,1998,10(11):1779-1790.
[6] JIN D,LI B S,DENG X W,WEI N. Plant COP9 signalosome subunit 5,CSN5[J]. Plant Science,2014,224:54-61.
[7] CUI K C,LIU M,KE G H,ZHANG X Y,MU B,ZHOU M,HU Y,WEN Y Q. Transient silencing of VvCSN5 enhances powdery mildew resistance in grapevine (Vitis vinifera)[J]. Plant Cell,Tissue and Organ Culture,2021,146(3):621-633.
[8] DENTI S,FERNANDEZ-SANCHEZ M E,ROGGE L,BIANCHI E. The COP9 signalosome regulates Skp2 levels and proliferation of human cells[J]. Journal of Biological Chemistry,2006,281(43):32188-32196.
[9] LOZANO-DUR?N R,ROSAS-D?AZ T,GUSMAROLI G,LUNA A P,TACONNAT L,DENG X W,BEJARANO E R. Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana[J]. The Plant Cell,2011,23(3):1014-1032.
[10] SHANG Y F,WANG K X,SUN S C,ZHOU J,YU J Q. COP9 signalosome CSN4 and CSN5 subunits are involved in jasmonate-dependent defense against root-knot nematode in tomato[J]. Frontiers in Plant Science,2019,10:1223.
[11] SINGH A K,YADAV B S,DHANAPAL S,BERLINER M,FINKELSHTEIN A,CHAMOVITZ D A. CSN5A subunit of COP9 signalosome temporally buffers response to heat in Arabidopsis[J]. Biomolecules,2019,9(12):805.
[12] LIAO X,LI M S,LIU B,YAN M L,YU X M,ZI H L,LIU R Y,YAMAMURO C. Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry[J]. Proceedings of the National Academy of Sciences of the United States of America,2018,115(49):E11542-E11550.
[13] MOYA-LE?N M A,STAPPUNG Y,MATTUS-ARAYA E,HERRERA R. Insights into the genes involved in ABA biosynthesis and perception during development and ripening of the Chilean strawberry fruit[J]. International Journal of Molecular Sciences,2023,24(10):8531.
[14] 鄭珍珍,陳雪雪,沈元月,黃蕓. 轉錄因子FabHLH148參與草莓果實的顏色發育[J]. 果樹學報,2022,39(8):1358-1367.
ZHENG Zhenzhen,CHEN Xuexue,SHEN Yuanyue,HUANG Yun. Transcription factor FabHLH148 is involved in the color development of strawberry fruit[J]. Journal of Fruit Science,2022,39(8):1358-1367.
[15] 楊潔. 丹參SmMYB36與SmCSN5蛋白的互作關系鑒定及其作用分析[D]. 楊凌:西北農林科技大學,2021.
YANG Jie. Identification and analysis of interaction between SmMYB36 and SmCSN5 protein in Salvia miltiorrhiza[D]. Yangling:Northwest A & F University,2021.
[16] MAO J X,WANG Y,WANG B T,LI J Q,ZHANG C,ZHANG W S,LI X,LI J,ZHANG J X,LI H,ZHANG Z H. High-quality haplotype-resolved genome assembly of cultivated octoploid strawberry[J]. Horticulture Research,2023,10(1):uhad002.
[17] GU T T,JIA S F,HUANG X R,WANG L,FU W M,HUO G T,GAN L J,DING J,LI Y. Transcriptome and hormone analyses provide insights into hormonal regulation in strawberry ripening[J]. Planta,2019,250(1):145-162.
[18] MUSAZADE E,LIU Y X,REN Y X,WU M,ZENG H,HAN S N,GAO X W,CHEN S H,GUO L Q. OsCSN1 regulates the growth and development of rice seedlings through the degradation of SLR1 in the GA signaling pathway[J]. Agronomy,2022,12(12):2946.
[19] 張夢柯. GmCSN5s的克隆及其調控大豆適應低磷脅迫的機制初探[D]. 廣州:華南農業大學,2019.
ZHANG Mengke. Clonging and charaterizatino of GmCSN5s functions in soybean adaptation to phosphorus deficiency[D]. Guangzhou:South China Agricultural University,2019.
[20] 吳紹華,張世鑫,鄧小敏,陳月異,田維敏. 橡膠樹COP9家族基因的克隆及表達分析[J]. 熱帶作物學報,2019,40(2):281-288.
WU Shaohua,ZHANG Shixin,DENG Xiaomin,CHEN Yueyi,TIAN Weimin. Gene cloning and expression analysis of the COP9 signalosome members in laticifer cells of rubber tree[J]. Chinese Journal of Tropical Crops,2019,40(2):281-288.
[21] 龔茵茵. CSN5通過調節AsA的穩態介導水稻鹽脅迫響應的分子機制[D]. 長沙:湖南大學,2020.
GONG Yinyin. The molecular mechanism of CSN5 mediates salt stress response in rice by regulating AsA homeostasis[D]. Changsha:Hunan University,2020.
[22] SHI B Z,HOU J Q,YANG J,HAN I J,TU D Y,YE S Q,YU J F,LI L J. Genome-wide analysis of the CSN genes in land plants and their expression under various abiotic stress and phytohormone conditions in rice[J]. Gene,2023,850:146905.
[23] ZHENG Q Z,ZHANG L,ZHANG Q,PANG Z Y,SUN Y,YIN Z,LOU Z Y. Discovery of interacting proteins of ABA receptor PYL5 via covalent chemical capture[J]. ACS Chemical Biology,2019,14(12):2557-2563.
[24] JIN D,WU M,LI B S,B?CKER B,KEIL P,ZHANG S M,LI J G,KANG D M,LIU J,DONG J,DENG X W,IRISH V,WEI N. The COP9 signalosome regulates seed germination by facilitating protein degradation of RGL2 and ABI5[J]. PLoS Genetics,2018,14(2):e1007237.
[25] BAI X X,HUANG X L,TIAN S X,PENG H,ZHAN G M,GOHER F,GUO J,KANG Z S,GUO J. RNAi-mediated stable silencing of TaCSN5 confers broad-spectrum resistance to Puccinia striiformis f. sp. tritici[J]. Molecular Plant Pathology,2021,22(4):410-421.
[26] HAN S N,LIU Y X,BAO A,ZENG H,HUANG G H,GENG M,ZHANG C Y,ZHANG Q,LU J M,WU M,GUO L Q. OsCSN1 regulates the growth of rice seedlings through the GA signaling pathway in blue light[J]. Journal of Plant Physiology,2023,280:153904.

