A Ratiometric Fluorescent Polystyrene Microsphere Hybrid Probe for Highly Selective Detection of Anthrax Markers
HU Runze, XU Chen, FANG Zhou, LI Ying*, MIN Hua
(1.School of Materials & Chemistry, University of Shanghai for Science and Technology, Shanghai 200093, China;2.Technology Transfer Center, Institute of Science and Technology Development,University of Shanghai for Science and Technology, Shanghai 200093, China)
Abstract: Eu(DBM)3Phen was firstly encapsulated into carboxylated polystyrene microspheres by the encapsulation method, and then the lanthanide luminescence center Tb3+ was introduced by coordination to obtain the fluorescent polystyrene microsphere hybrid probe Tb-PS@Eu(DBM)3Phen with dual emission centers. The results indicated that Tb-PS@Eu(DBM)3Phen has excellent stability, dispersibility and fluorescence properties. In addition, by further investigating the fluorescence sensing properties of the probe molecule on 2,6-pyridinedicarboxylic acid(DPA), it was found that Tb-PS@Eu(DBM)3Phen could produce a significant enhancement with the present of DPA, which might be due to the coordination of terbium ions on the surface of DPA and polystyrene microspheres,which in turn affected the energy transfer process between the ligand-rare earths, resulting in Tb-PS@Eu-(DBM)3Phen's fluorescence enhancement. Meanwhile, Tb-PS@Eu(DBM)3Phen has strong selectivity and anti-interference ability for DPA, which is expected to be used as a potential fluorescent probe for the recognition of DPA.
Key words: lanthanide complexes; fluorescent microspheres; DPA; ratiometric fluorescent sensing
1 Introduction
Despite that various means of preventing anthrax, it still represents a considerable public health hazard to the world community[1-3]. Anthrax is known to be an infectious disease caused by the bacterium Bacillus anthracis, which can cause fatal infection if more than 104spores are inhaled in a 36 h period.Bacillus anthracis belongs to the genus bacillus aerobicus which is a rod-shaped, gram-positive bacterium that can not only be ubiquitous in soil but also persist for decades, surviving for long periods of time even under extreme adverse conditions such as high temperatures, strong UV radiation, and strong acidic or alkaline conditions[4-5]. In addition, once the surrounding environment meets its growth requirements, anthrax bacilli are activated and spread rapidly. Humans can contract anthrax in a number of ways, including inhalation of anthrax spores, contact with anthrax-contaminated materials, and consumption of inadequately heated meat from sick animals resulting in intestinal anthrax. Therefore, anthracis is considered a potentially extremely lethal biological warfare agent and has received widespread international attention[6]. As a major component of bacterial spores, 2,6-pyridinedicarboxylic acid(DPA) accounts for 5%-15% of the dry mass of spores and is not found in other natural or synthetic materials, so it can be used as a typical anthrax biomarker[7-8].Therefore, exploring an efficient and accurate DPA assay is of great importance.
Several methods have been developed for the detection of DPA, including surface-enhanced Raman spectroscopy(SERS)[9], polymerase chain reaction(PCR)[10], superparamagnetic cross-flow immunoassay(LFA)[11], gas chromatography/mass spectrometry(GC/MS)[12], and electrochemical detection[13].However, traditional detection methods, such as HPLC and GC/MS, require specialized operators,complex sample preparation and expensive instruments, and LFA and PCR require more demanding procedures and expensive chemicals, which may be a barrier to practical application and are not suitable for commercial use. In contrast, fluorescence detection has become a more economically competitive option that attributed to its low cost, ease of operation,high selectivity and sensitivity, fast response time and real-time monitoring[14-16].
Due to the unique spectral properties of rare earth ions, such as long fluorescence lifetime, large Stokes shift and narrow emission band, fluorescence sensors based on rare earth ions have been developed rapidly[17-20]. In particular, two rare earth elements, Eu3+and Tb3+, located in the visible region.In recent years, nanomaterials functionalized based on these two rare earth ions have been used for the detection of DPA[21-23]. However, as a single emission sensor to detect DPA is susceptible to interference from background light, temperature, instrumentation,and other external conditions that affect the results of detection[24]. Fortunately, ratiometric fluorescent probes can be color adjusted and self-calibrated by measuring fluctuations in the ratio of fluorescence intensity at two wavelengths, thus eliminating environmental or instrumental interference and increasing the accuracy of detection results and are considered ideal tools for building sensing platforms[25-28]. Fluorescent microspheres[29-33]with their stable morphological structure and stable and efficient luminescence efficiency[34-35], have been used in labeling, intelligence analysis, detection immobilized enzymes,immunological medicine, and high-throughput drug screening[36-40]. In particular, fluorescent microspheres using polystyrene microspheres as a substrate have many advantages of polystyrene microspheres themselves. For instances, large specific surface area, good stability, strong surface reactivity,and easy surface functionalization. In addition, the surface of microspheres can relate to different bioactive macromolecules, which in turn can be widely used in biomarkers, fluorescent probes, bioimaging,fluorescent sensors and immunoassays[41]. Therefore,in this thesis, a ratiometric fluorescent probe Tb-PS@Eu(DBM)3Phen was synthesized using Eu3+as the reference fluorescence, introducing europium complexes into the interior of polystyrene microspheres in the form of encapsulation, and then ligating with terbium ions through carboxyl groups on the surface of polystyrene microspheres. Compared with measurements performed at a single wavelength, it has strong anti-interference properties and avoids the influence of external factors such as the instrument, the dose of the detector and temperature on the results, enabling the sensitive detection of the bacillus anthracis biomarker DPA.
2 Experiment
2.1 Preparation of Tb-PS@Eu(DBM)3Phen
2.1.1 Experimental Reagents
NaHCO3, MMA, K2S2O8, anhydrous ethanol,benzoic acid, isophthalic acid, homo-phthalic acid,glutamine, glycine, 3,4-pyridinedicarboxylic acid, 2,4-pyridinedicarboxylic acid, 3,5-pyridinedicarboxylic acid MAA, LnCl3·6H2O, L-Aspartic acid.
2.1.2 Preparation Process
(1) Purification of styrene
To reduce the polymerization loss of styrene monomer, polymerization inhibitors are often added to styrene. Therefore, it is necessary to purify styrene with alkaline alumina before use. We take an appropriate amount of alkaline alumina in the chromatographic column, add 100 mL of styrene in the column to remove the polymerization inhibitor. The purified styrene is put in a dry and clean reagent bottle, and stored in the refrigerator at 2-8 ℃.
(2)Preparation of rare earth complex Eu(DBM)3-Phen
6 mmol of dibenzoylmethane, 2 mmol of 1-10 anhydrous phloroglucinol and 6 mmol of sodium hydroxide were mixed into 40 mL of anhydrous ethanol and placed in an oil bath to dissolve with stirring.When it was warmed up to 50 ℃, 20 mL of ethanol solution dissolved with 2 mmol EuCl3·6H2O was added slowly dropwise and the mixture was refluxed at 50 ℃ for another 2 h. The mixture was left to reaction for 3 h and then filtered by brinell funnel extraction and washed several times with ethanol/water. The resulting solid powder was dried at 60 ℃for 12 h.
(3) Synthesis of carboxylated fluorescent microspheres PS@Eu(DBM)3Phen
Carboxylated fluorescent polystyrene microspheres were prepared using soap-free emulsion polymerization method and embedding method. First,water(32 mL), NaHCO3(0.24 mg), methacrylate(0.85 mL containing Eu(DBM)3Phen(40 mg)) and styrene(2 mL) were placed in a 100 mL three-neck flask. After purging with nitrogen for 30 min, the temperature was gradually increased to 80 ℃ and 2 mL(0.01 g) of aqueous potassium persulfate solution was added. Then, after 30 min reaction, 0.2 mL of methacrylic acid and 2 mL(0.01 g) of aqueous potassium persulfate were added. Finally, the reaction was continued for 10 h and cooled to room temperature.
(4) Preparation of fluorescent microspheres Tb-PS@Eu(DBM)3Phen
At the end of the above experiment, TbCl3·6H2O(0.1 g) was added to continue the reaction for 1 h. Finally, the reaction was cooled to room temperature and washed alternately with alcohol and water and redispersed in ethanol(Fig.1).
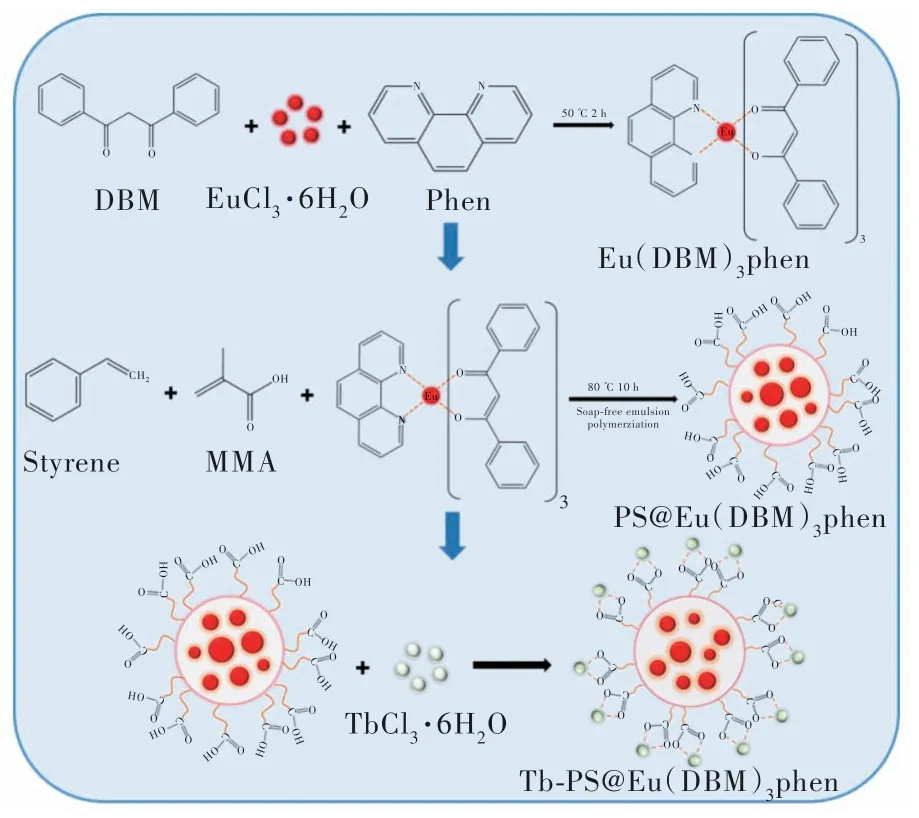
Fig.1 Experimental flow chart of Tb-PS@Eu(DBM)3Phen
(5) Fluorescence sensing detection of DPA by Tb-PS@Eu(DBM)3Phen
30 mg of Tb-PS@Eu(DBM)3Phen powder was dissolved in 10 mL of anhydrous ethanol and sonicated for 5 min to disperse uniformly to form a clear suspension. Under the same experimental conditions,the fluorescence of Tb-PS@Eu(DBM)3Phen was also measured at the excitation wavelength of 280 nm.
In the anti-interference experiments, the Tb-PS@Eu(DBM)3Phen solutions were prepared in the same way as the above experiments, except that 100 μL of other substances with similar structures of different species(concentrations of 20 μmol/L)were added, respectively, including benzoic acid(BEN), isophthalic acid(IPA), homo-phthalic tricarboxylic acid(BTC), L-aspartic acid (L-ASP),glutamic acid(GLC), glycine(GLY), 3,4-pyridinedicarboxylic acid(3,4-PCA), 2,4-pyridinedicarboxylic acid(IA), and 3,5-pyridinedicarboxylic acid(3,5-PCA).
2.2 Performance and Characterization of Tb-PS@Eu(DBM)3Phen
The Fourier Transform Infrared Spectroscopy(FT-IR) was measured by the American Nexus 912 AO446 spectrometer, and the solid samples were prepared by KBr tablet technology. The fluorescence intensity was measured by the Japanese RF-5301PC spectrophotometer, and the xenon lamp was used as the excitation light source. X-ray photoelectric spectrometry(XPS) was measured by the American Thermo Scientific K-Alpha spectrometer. The ultraviolet-visible spectrum(UV-Vis) was measured by the American Lambda 750 spectrometer, and the sample was deionized water as the solvent. Scanning electron microscope(SEM) measurements were conducted on a Sigma 300 at an accelerating voltage of 20 kV. Fluorescence lifetime was measured by Edinburgh FLS1000.
3 Results and Discussion
3.1 Structural Characterization of Tb-PS@Eu-(DBM)3Phen
The formation of Tb-PS@Eu(DBM)3Phen can be verified by the FT-IR spectrum in Fig.2. As can be seen that an absorption band at 1 740 cm-1is observed in the Tb-PS@Eu(DBM)3Phen spectrum,which can be attributed to the C=O absorption peak. Also, the methylene absorption peak of polystyrene microspheres appears at 2 924 cm-1, indicating the successful polymerization of methacrylic acid with polystyrene. In addition, 1 606 cm-1could be contributed to the skeletal vibrational absorption peak of the benzene ring. The characteristic absorption of C—O located at 1 453 cm-1and 1 492 cm-1,respectively, indicating that the complex Eu(DBM)3-Phen has been successfully introduced into the carboxylated polystyrene microsphere table. The Tb—O stretching vibrational peak appears in the fingerprint region(450-500 cm-1) indicating the successful grafting of Tb onto the surface of polystyrene microspheres[42-43].
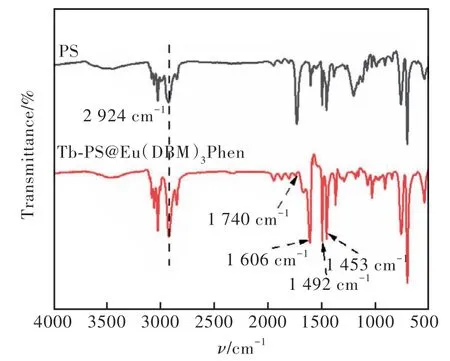
Fig.2 FT-IR spectra of Tb-PS@Eu(DBM)3Phen and PS
Further analysis of XPS results of Tb-PS@Eu-(DBM)3Phen as shown in Fig.3(a). The characteristic peaks of C 1s, N 1s, O 1s, Tb 3d, and Eu 3d appear at 283.08, 316.08, 531.4, 1 257.7, 1 134.94 eV. This indicates that Tb-PS@Eu(DBM)3Phen is composed of five elements: carbon, nitrogen, oxygen,terbium and europium, with elemental contents of 91.52%, 1.16%, 6.87%, 0.19% and 0.26%, which also further confirmed the successful binding of Tb3+and Eu3+to the PS. In addition, the high-resolution XPS spectra indicated that the Tb-PS@Eu-(DBM)3Phen in the carbon element was presented as C=C(284.7 eV) and C—O(286.2 eV) and O=C=O(288.4 eV)(Fig.3(b))[44-45], and the nitrogen element was presented as N—Eu(400.4 eV)(Fig.3(c))[46], while oxygen elements are presented as C=O(531.3 eV), C—O/C—OH(532.2 eV) andM—O(M=Tb, Eu) groups(530.5 eV)(Fig.3(d))[47].Fine spectra of Tb 3d and Eu 3d were also observed(Fig.3(e)-(f)), and XPS analysis results were consistent with FT-IR spectra.

Fig.3 (a)XPS full spectrum of Tb-PS@Eu(DBM)3Phen. (b)C 1s fine spectra. (c)N 1s fine spectra. (d)O 1s fine spectra. (e)Tb 3d fine spectra. (f)Eu 3d fine spectrum.
3.2 Morphological Characterization of Tb-PS@Eu(DBM)3Phen
As illustrated in Fig.4, the SEM image of the scale 2 μm Tb-PS@Eu(DBM)3Phen, it can be observed that the prepared polystyrene fluorescent microspheres have a spherical morphology and uniform microsphere size with good dispersion. The SEM image of PS@Eu(DBM)3Phen is revealed in the Fig.4(a). According to the typical preparation method,the polystyrene microspheres carrying carboxyl groups on the surface were synthesized by the polymerization method of soap-free emulsion. And the fluorescent polystyrene microspheres PS@Eu(DBM)3-Phen were prepared by introducing the complex Eu-(DBM)3Phen in the encapsulation form during the polymerization process. It can be observed that the prepared microspheres have a uniform size of 300 nm and are well dispersed. Finally, the ratiometric fluorescent polystyrene microspheres Tb-PS@Eu-(DBM)3Phen with dual luminescence centers were obtained by using the carboxyl groups on the surface ligated with terbium ions, and the particle size was increased to 350 nm (Fig.4(b)).
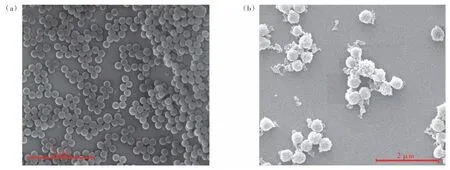
Fig.4 SEM images of PS@Eu(DBM)3Phen(a) and Tb-PS@Eu(DBM)3Phen(b)
3.3 Fluorescence Properties Analysis of Tb-PS@Eu(DBM)3Phen
The fluorescence excitation and emission spectra of PS@Eu(DBM)3Phen were given in Fig.5(a).When excited at 294 nm, four narrow characteristic emission spectra exhibit the 4f→4f transitions of Eu3+(5D0→7FJ,J=1-4), which located at 596, 611, 651,703 nm, respectively. The most intense emission peak at 611 nm is ascribed to5D0→7F2transition,and it induced the red emissions. PS@Eu(DBM)3-Phen can be observed to exhibit a bright red color under a 365 nm UV lamp. Fig.5(b) displays the fluorescence excitation and emission spectra of Tb-PS@Eu(DBM)3Phen, and it can be observed that the optimal excitation wavelength becomes 280 nm, grafted onto the surface of polystyrene microspheres.
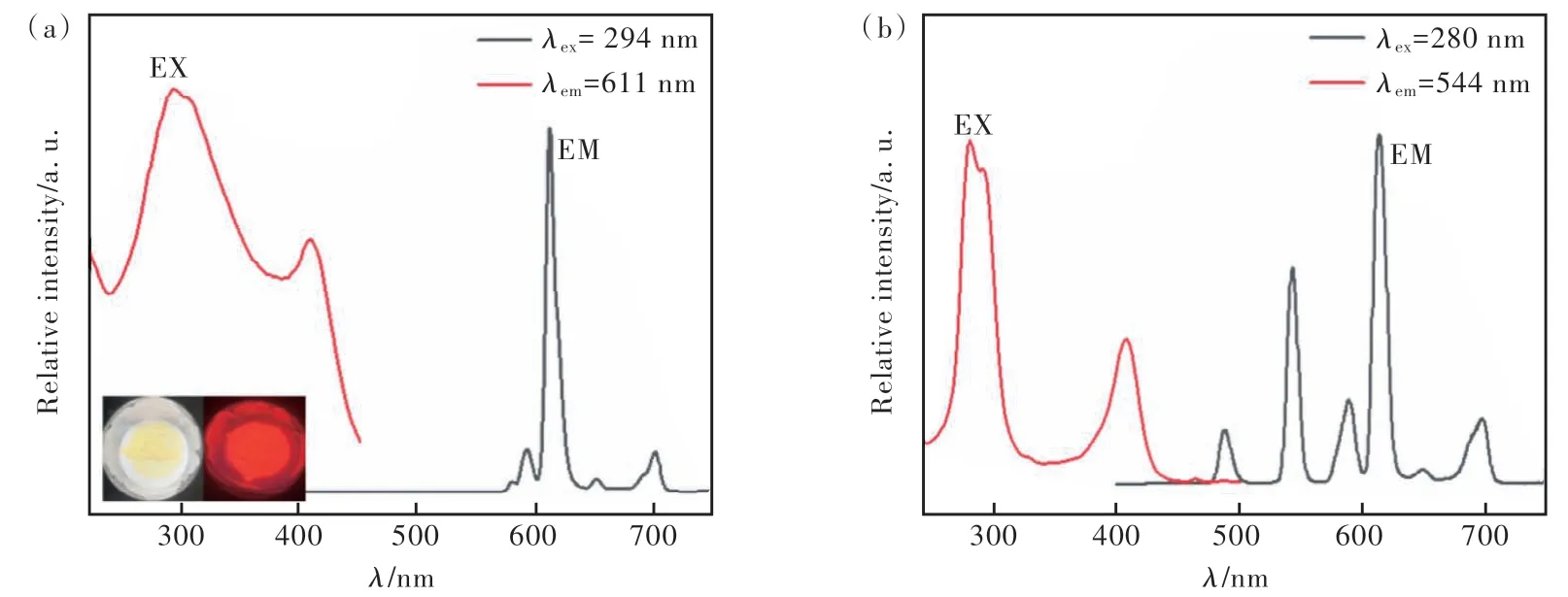
Fig.5 Fluorescence emission patterns of PS@Eu(DBM)3Phen(a) and Tb-PS@Eu(DBM)3Phen(b)
3.4 Sensing Detection of DPA by Tb-PS@Eu-(DBM)3Phen
(1) Detection feasibility analysis
The feasibility of detecting DPA was investigated by comparing the fluorescence emission profiles of Tb-PS@Eu(DBM)3Phen and Tb-PS@Eu(DBM)3-Phen + DPA. As exhibited in Fig.6, the fluorescence emission intensity at 615 nm was less changed after the addition of DPA molecules to Tb-PS@Eu(DBM)3Phen, and the fluorescence emission intensity at 544 nm was effectively enhanced.Meanwhile, the change of the probe from red to green could be clearly observed by the naked eye under the UV lamp irradiation, and these results confirmed the good feasibility of the probe in the detection of DPA.
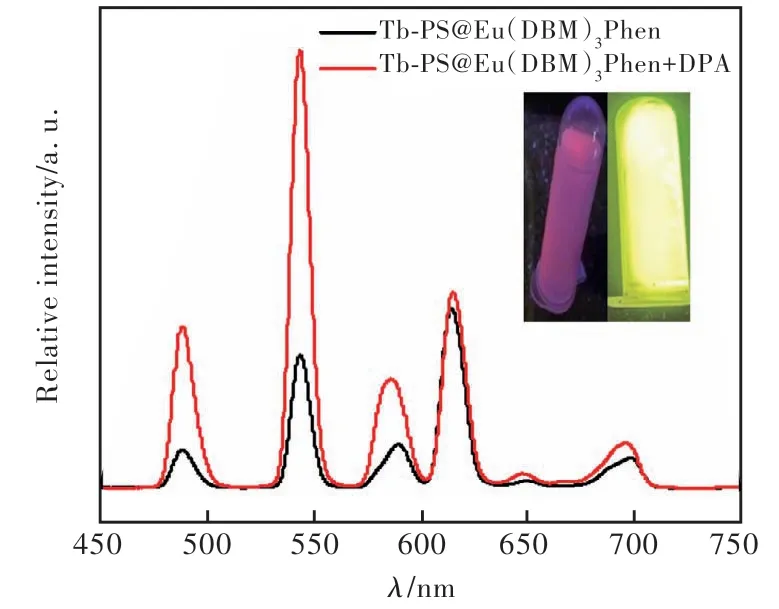
Fig.6 Fluorescence emission pattern of Tb-PS@Eu(DBM)3-Phen before and after the addition of PA
(2) Optimization of the detection conditions
The optimal detection conditions were obtained by optimizing the two parameters (equilibration time and temperature) that affect the detection efficiency,as shown in Fig.7. The concentration of the detector DPA was uniformly controlled as 20 μmol/L. Firstly, the effect of equilibration time corresponding to the fluorescence intensity effect was investigated, as shown in the figure, the fluorescence intensity of the probe Tb-PS@Eu(DBM)3Phen varied almost 0 within 0-20 min, indicating that the prepared probe has good time stability, as shown in Fig.7(a). Next, the temperature stability of the probe was investigated,as shown by Fig.7(b), the fluorescence intensity of the probe Tb-PS@Eu( DBM)3Phen decreased with the gradual increase of temperature within 10-45 ℃.Therefore, for the accuracy of the measurement we chose the transient detection at room temperature conditions.
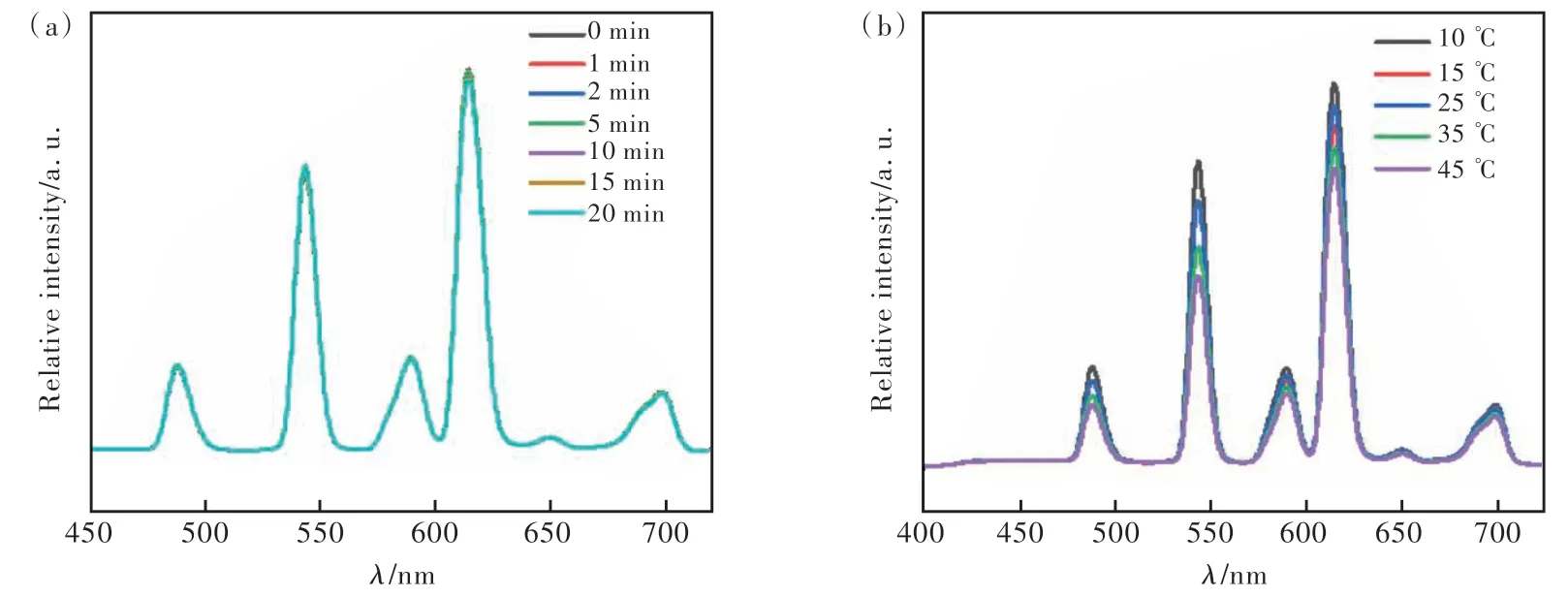
Fig.7 Effect of temperature and time on the fluorescence intensity of Tb-PS@Eu(DBM)3Phen
(3) Titration of DPA concentration and calculation of detection limit
Sensitivity is an important factor affecting the effect of fluorescent probes, therefore, in this thesis,the fluorescence emission spectra of Tb-PS@Eu-(DBM)3Phen were measured after adding different concentrations of DPA solution.
The fluorescence emission spectra of Tb-PS@Eu-(DBM)3Phen after the addition of different concentrations of DPA solution were analyzed, and the results were descripted in Fig.8(a). Obviously, when the concentration of DPA solution was in the range of 1-100 μmol/L, the fluorescence intensity at 544 nm was gradually enhanced with the increase of DPA concentration, and the fluorescence intensity at 615 nm was almost unchanged. In addition, we found that when the DPA concentration was in the range of 20-70 μmol/L, there was a good linear relationship betweenI615/I544and DPA concentration which can be calculated by the Stern-Volmer equation as
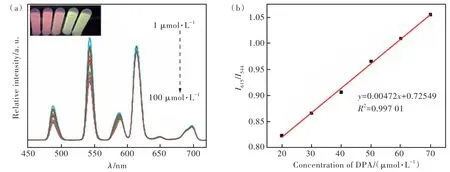
Fig.8 The emission spectra of Tb-PS@Eu(DBM)3Phen at different concentrations of DPA(a) and the linear relationship between peak fluorescence intensity ratio and DPA concentration(b)
whereCis the concentration of DPA,KSVis the Stern-Volmer constant, andI615andI544are the fluorescence intensity at 615 nm and 544 nm before and after the addition of DPA, respectively. As shown in Fig.8(b), the regression equation was obtained asI615/I544=0.00472C+0.72549(R2=0.997 01) by linear fitting. The limit of detection(D) of the probe was calculated from the 3σequation to be as low as 1.32 μmol/L:
σis the standard deviation obtained from 20 consecutive scans of the blank solution.Sis the slope of the linear equation. The good linear correlation and low detection limits imply that Tb-PS@Eu(DBM)3-Phen sensor has higher sensitivity and excellent detection capability for DPA.
(4) Specificity and selectivity of detection
Specificity and selectivity are also another important indicator of the effectiveness of fluorescent probes. The selectivity of the Tb-PS@Eu(DBM)3-Phen probe for DPA was explored by selecting several other analogues including BEN, IPA, BTC, LASP, GLC, GLY, 3,4-PCA, IA, and 3,5-PCA. As illustrated in Fig.9(a), several other substances exhibited different degrees of fluorescence enhancement responses. However, the fluorescence enhancement of DPA was obviously higher than that of several other substances(Fig.9(b)). This may be attributed to the terbium ion on the surface of the probe Tb-PS@Eu(DBM)3Phen has the strongest binding ability to DPA(Findicates the fluorescence intensity after the addition of different substances, andF0represents the fluorescence intensity of the Tb-PS@Eu(DBM)3Phen at 544 nm). Therefore, these results suggest that Tb-PS@Eu(DBM)3-Phen can be considered as a potential fluorescent sensor with good specificity and selectivity.
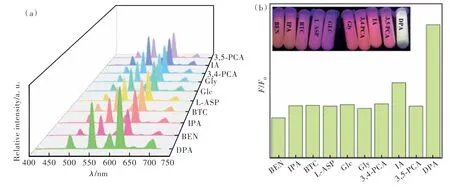
Fig.9 The emission spectra of Tb-PS@Eu(DBM)3Phen after adding different substances(a) and the histogram of fluorescence change after adding different substances(b)
(5) Detection mechanism
The energy transfer between the ligand and the central ion is the main reason for the fluorescence enhancement mechanism. The possible mechanism of fluorescence enhancement for Tb-PS@Eu(DBM)3-Phen by DPA may be attributed to the antenna effect. Since the coordination between PS@Eu(DBM)3-Phen and Tb3+in Tb-PS@Eu(DBM)3Phen is unsaturated, water molecules occupy the empty coordination center of Tb3+, leading to the quenching emission of Tb3+. The organic ligand DPA with β-diketone structure can chelate to the central Tb3+ions by coordinating bond as a co-ligand(Fig.10(d)). In addition, the fluorescence lifetime of Tb-PS@Eu(DBM)3-Phen has been tested to further illustrated that the introduction of DPA can improve the fluorescence properties of the samples(Fig.10(b)-(c)).
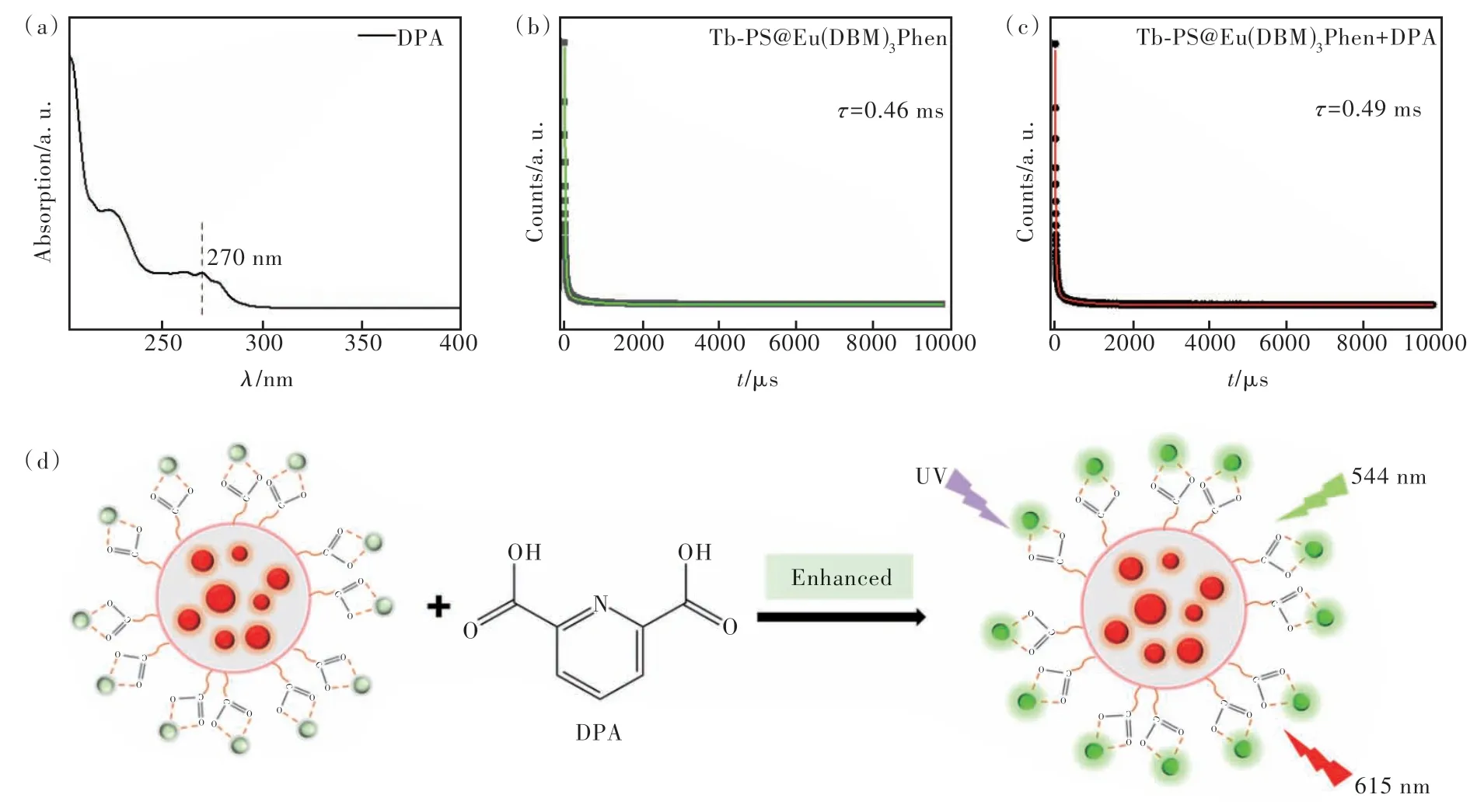
Fig.10 (a)UV-Vis spectrum of DPA. Fluorescence lifetime of Tb-PS@Eu(DBM)3Phen(b) and Tb-PS@Eu(DBM)3Phen + DPA(c). (d)Mechanism of DPA detection by Tb-PS@Eu(DBM)3Phen.
4 Conclusion
In summary, we constructed a lanthanide-based ratiometric fluorescent probe Tb-PS@Eu(DBM)3-Phen. By further investigating the fluorescence sensing performance of the probe molecule on DPA, it was found that DPA can produce a significant enhancement effect on the fluorescence of Tb-PS@Eu-(DBM)3Phen. Meanwhile, Tb-PS@Eu(DBM)3Phen has strong selectivity and anti-interference ability for DPA with good linearity in 20-70 μmol/L and low detection limit 1.32 μmol/L, which is potential to develop into a fluorescent sensor for visual detection of DPA .
Response Letter is available for this paper at:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20230049.

