反相高效液相色譜法測(cè)定頭孢地尼及制劑中的聚合物類(lèi)雜質(zhì)
楊倩 張琳 曹曉云 唐素芳
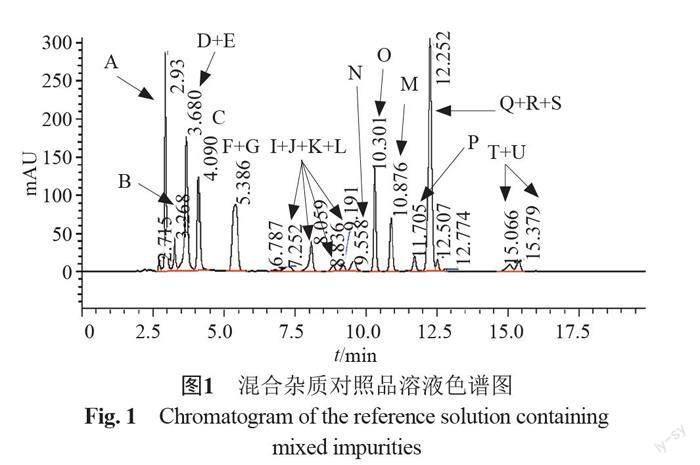
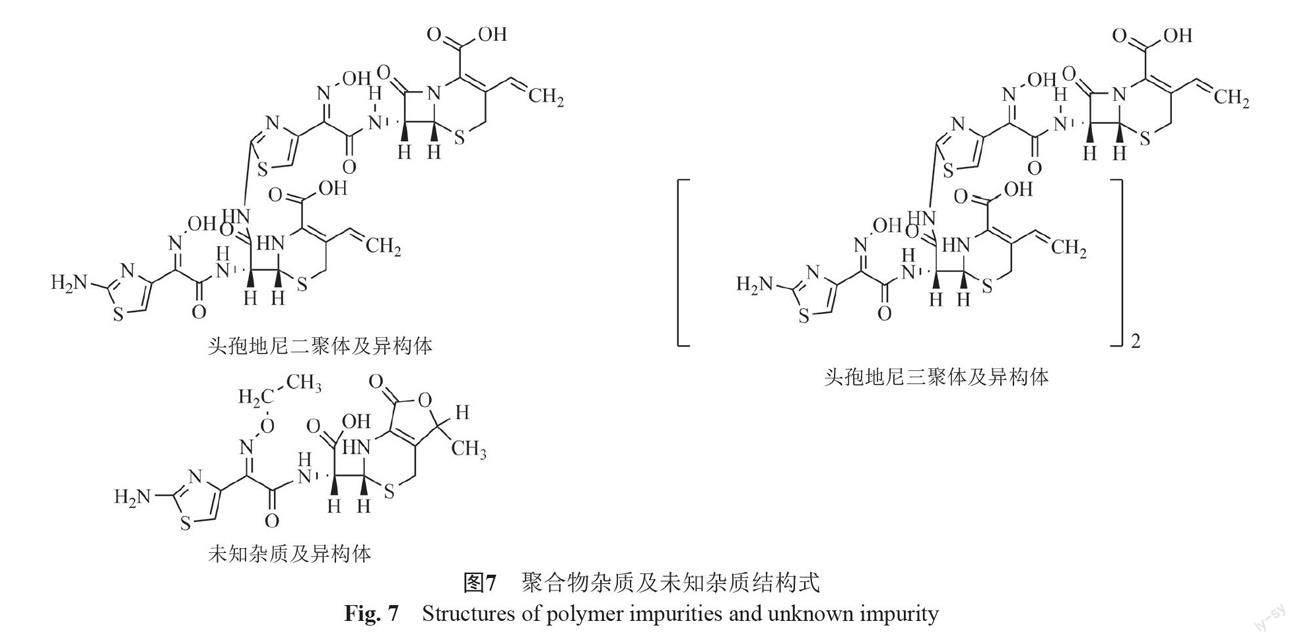
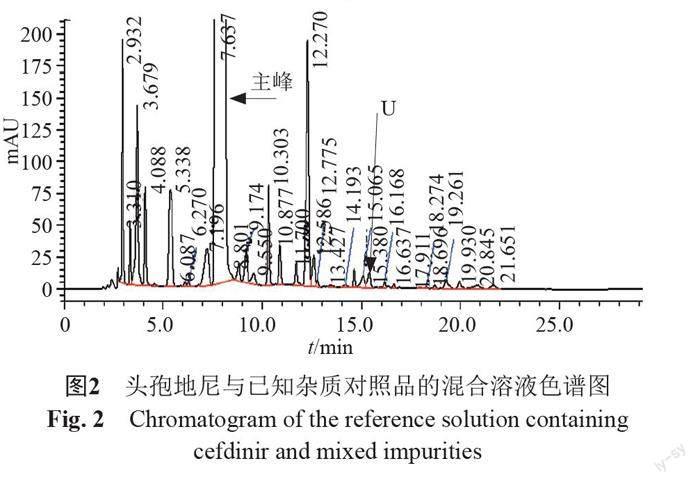
摘要:目的 建立反相高效液相色譜法測(cè)定頭孢地尼及制劑中的聚合物雜質(zhì)。方法 以CAPCELL PAK C18 MGⅡ?yàn)樯V柱(250 mm×4.6 mm,5 μm),0.25%四甲基氫氧化銨溶液(pH 5.5)[每1000mL中加入0.1 mol/L乙二胺四乙酸二鈉溶液0.4 mL]-甲醇-乙腈為流動(dòng)相系統(tǒng),梯度洗脫,建立了頭孢地尼聚合物類(lèi)雜質(zhì)的分析方法并進(jìn)行了方法學(xué)驗(yàn)證。采用二維液質(zhì)聯(lián)用法對(duì)檢出的聚合物類(lèi)雜質(zhì)進(jìn)行定性分析。結(jié)果 在該色譜條件下頭孢地尼及已知雜質(zhì)均與頭孢地尼聚合物間分離良好,頭孢地尼檢測(cè)濃度的線性范圍為0.30~30.1 μg/mL(r=1.0000),方法的檢測(cè)限與定量限分別為1.5 ng和4.5 ng。所建立的方法能夠檢出頭孢地尼二聚體,頭孢地尼二聚體的多個(gè)同分異構(gòu)體及頭孢地尼三聚體。結(jié)論 本方法專(zhuān)屬性好,靈敏度高,耐用性好,可用于頭孢地尼及其制劑中聚合物雜質(zhì)的控制。
關(guān)鍵詞:頭孢地尼;聚合物;雜質(zhì);反相高效液相色譜法;二維液質(zhì)聯(lián)用
中圖分類(lèi)號(hào):R978.1 ? ? ? ?文獻(xiàn)標(biāo)志碼:A
Determination of polymer impurities in cefdinir and preparations by RP-HPLC
Yang Qian, Zhang Lin, Cao Xiao-yun, and Tang Su-fang
(Tianjin Institute for Drug Control, Tianjin 300070)
Abstract Objective To establish a reverse high-performance liquid chromatography (HPLC) for the separation and determination of polymers in cefdinir and its preparations. Methods The method was performed by using CAPCELL PAK C18 MG Ⅱ column (250 mm×4.6 mm,5 μm). The mobile phase was 0.25% tetramethyl ammonium hydroxide (pH5.5) containing 0.1 mol/L disodium EDTA solution (0.4 mL per 1000 mL)-acetonitrile-methanol. The gradient elution was used. The method was validated, and the polymer impurities were analyzed by the two dimensional liquid chromatography-mass spectrometry method. Results Cefdinir, the identified impurities and polymers were separated effectively. The linear range of cefdinir was 0.30~30.1 μg/mL (r=1.0000). The limit of detection was 1.5 ng and the limit of quantitation was 4.5 ng. Cefdinir dimer, isomers of cefdinir dimer, and cefdinir trimer can be detected by the new method. Conclusion The established RP-HPLC method with good robustness is specific and sensitive. Therefore, it can be used for the control of polymer impurities in the pharmaceutical preparations.
Key words Cefdinir; Polymers; Impurities; RP-HPLC; 2D-LC-MS
頭孢地尼為半合成第三代β-內(nèi)酰胺類(lèi)抗生素,由日本藤澤藥品工業(yè)公司研發(fā),1991年首次在日本上市,2001年國(guó)產(chǎn)仿制藥獲準(zhǔn)上市。頭孢地尼通過(guò)抑制細(xì)菌細(xì)胞壁的合成產(chǎn)生抗菌作用,對(duì)革蘭陽(yáng)性菌和陰性菌均有抗菌活性,在臨床上廣泛應(yīng)用于內(nèi)外科、皮膚科和婦產(chǎn)科等敏感菌導(dǎo)致的感染。不良反應(yīng)輕微,以消化道反應(yīng)和過(guò)敏反應(yīng)最為常見(jiàn)。而過(guò)敏反應(yīng)正是β-內(nèi)酰胺類(lèi)抗生素最受關(guān)注的一類(lèi)不良反應(yīng),引發(fā)過(guò)敏反應(yīng)的過(guò)敏原是β-內(nèi)酰胺類(lèi)抗生素中的聚合物雜質(zhì)[1-3]。該類(lèi)雜質(zhì)在藥品的生產(chǎn)、貯藏、運(yùn)輸過(guò)程中均有可能產(chǎn)生,因此《中國(guó)藥典》從2000年版開(kāi)始對(duì)其進(jìn)行控制。……

