Impact of coronavirus disease 2019 on semen parameters
Dear editor,
Severe acute respiratory syndrome coronavirus-2(SARS-CoV-2),the virus responsible for the coronavirus disease 2019(COVID-19)pandemic,has been associated with hypogonadism in patients with acute infection[1].Angiotensin-converting enzyme 2(ACE2)receptor is regarded as the viral binding site that facilitates entry into host cells[2].Therefore,cells that express ACE2,such as spermatogonia,Leydig,and Sertoli cells,may be susceptible to SARS-CoV-2 infection[2].Testicular damage may possibly be caused directly by viral invasion or secondary to the generalized inflammatory response to COVID-19[3].Studies have demonstrated that viral shedding in the semen can take place during the recovery phase,even though SARSCoV-2 is not present in the semen of all men with COVID-19[1,4].Herein,we describe the first case report of a male with longitudinal follow-up who experienced reversible cryptozoospermia after COVID-19.
In July 2020,a 33-year-old male presented to the fertility clinic with a chief complaint of infertility.He had no history of genitourinary surgery,cryptorchidism,or scrotal trauma.A past chlamydia infection had been treated without sequelae.His reproductive history was notable for a pregnancy with a prior female partner.His current partner is a 32-year-old female with regular menses and normal gynecological evaluation.The patient reported no issues with erectile,ejaculatory,or orgasmic function.
A urologist had seen him in March 2020.His semen analysis(SA)at that time was notable for oligospermia(Table 1).A scrotal ultrasound showed a 15.4 mL right testicle,a 10.6 mL left testicle,and a small left varicocele.The patient was given clomiphene citrate 25 mg daily,an intervention not supported by current guidelines[5].
The patient became febrile to 39°C with fatigue and cough from the end of April to beginning of May 2020.The patient was quarantined at home and recovered within 2—3 weeks,with a presumptive diagnosis of COVID-19.He did not require any medical intervention and used nonsteroidal anti-inflammatory drugs for fever control.He never developed scrotal or pelvic pain.
In July 2020,he had a repeat SA that demonstrated cryptospermia with no sperm on examination of the neat semen sample and less than two sperm per high-power field(sperm/hpf)in the centrifuged specimen(Table 1).At that point he was referred to Weill Cornell Medicine for further management. On physical examination,the patient appeared well-virilized and had bilaterally descended testicles with the right testicle measuring 18 mL and the left testicle 12 mL by orchidometer.Grade I and II varicoceles were noted on the right and left sides,respectively.The patient was still taking 25 mg clomiphene daily.Laboratory studies were notable for morning total testosterone 1089 ng/dL,estradiol 58 pg/dL,and follicle stimulating hormone 28 mIU/mL.His SARS-CoV-2 antibody test was positive 2 months after his symptomatic infection.His clomiphene was stopped and repeat SA in October 2020 demonstrated substantial recovery of his SA parameters to his baseline oligospermia(Table 1).
To our knowledge,this is the first report documenting reversible cryptozoospermia after COVID-19.SARS-CoV-2 remains detectable in the semen of recovering patients for days to weeks[1].Our case report suggests that the effects of SARS-CoV-2 on spermatogenesis may linger months after clinical recovery,as would be expected given the long duration of spermatogenesis.Our patient was infected in April 2020,his symptoms resolved by mid-May 2020 and after a substantial symptom-free period,his SA in July 2020 demonstrated cryptospermia.Thus,the effect of COVID-19 in his semen appears to last significantly longer than the acute phase of his infection.The patient’s recent semen parameters in October 2020,approximately 5 months after acute symptoms,were restored back to his previous baseline.
Numerous viruses such as Zika,Ebola,herpes simplex,Epstein Barr,Mumps,and others are known to cause orchitis or even lead to male infertility[6].This may be mediated by direct damage to spermatozoa,abnormal sex-hormone secretion, or increased intratesticular pressure during orchitis that limits testicular blood flow[7].Mumps and human immunodeficiency virus constitute two examples of virus-induced testis damage that can impair hormone secretion and spermatogenesis[6].Similarly,SARS-CoV-2 has been identified as a cause of viral orchitis and possibly male infertility[4].
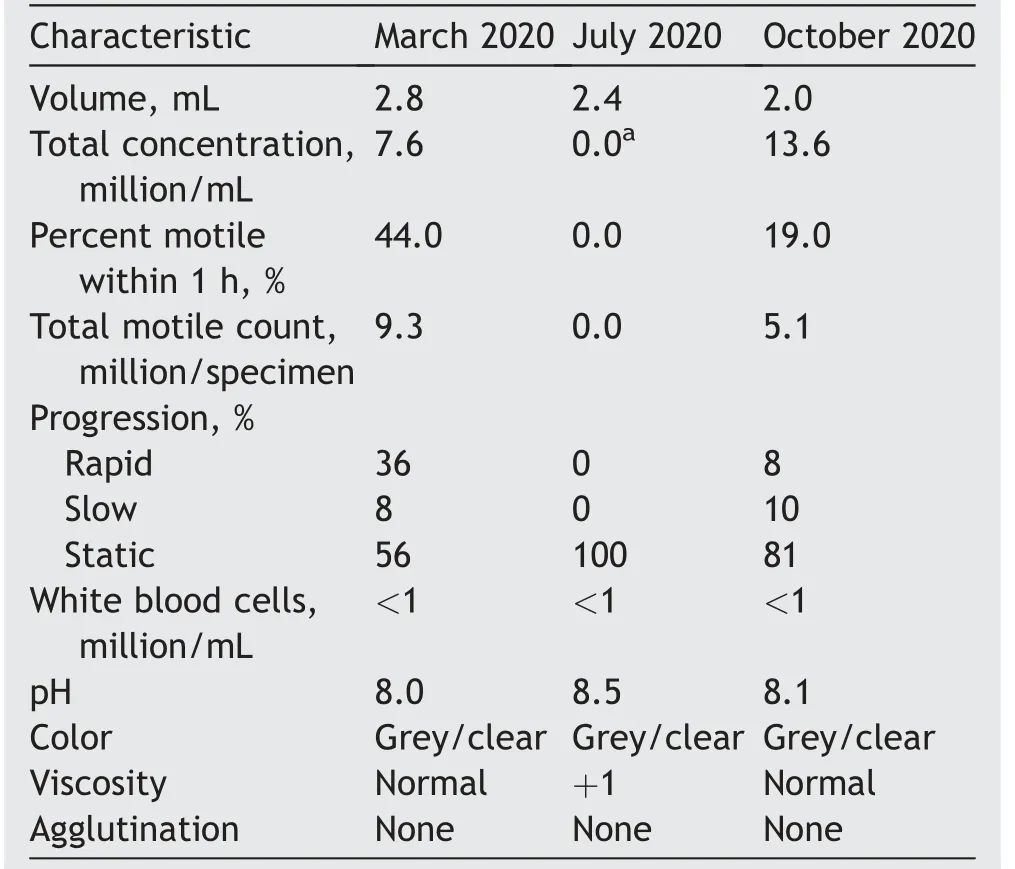
Table 1 Semen analysis from March 2020 to October 2020.
The ACE2 receptor is widely expressed in various organs,including the lungs,the gastrointestinal system,and the testes.There are two possible mechanisms by which SARSCoV-2 penetrates the blood-testis barrier and enters the reproductive cells and their surrounding environment.It has been reported that SARS-CoV-2 uses ACE2 as well as the transmembrane serine protease 2(TMPRSS2)to enter host cells.Wang and Xu[8]demonstrated that TMPRSS2 exists in spermatogonia and spermatids,while ACE2 is expressed in spermatogonia,Leydig,and Sertoli cells.Therefore,a mechanism for a direct effect of the virus on spermatogenesis is possible,but orchitis appears to be present in less than 3%of COVID-19[9].Our observations suggest that a more common effect of the virus may be due to the systemic effect of SARS-CoV-2,associated with febrile viral infections.
SARS-CoV-2 infection of the reproductive system could affect gonadal function.Ma et al.[7]compared the sexrelated hormones between 81 reproductive-aged men with COVID-19 and 100 age-matched healthy men.They found that serum luteinizing hormone was significantly increased and the ratio of testosterone to luteinizing hormone was dramatically decreased in males with COVID-19[7].Although this study was limited by a small sample size and a lack of semen data,these results put forth the possibility of decreased gonadal function in the setting of COVID-19.This is consistent with the prolonged recovery of semen parameters in the index case relative to the cessation of symptoms.Furthermore,Holtmann et al.[10]showed that a mild COVID-19 infection is unlikely to affect testicular function,but moderate severity infections could impair semen parameters.These findings suggest that the potentially deleterious effects of COVID-19 on the male reproductive system might outlast the acute phase of the illness and thus have negative ramifications for fertility.
Although early evidence suggests that SARS-CoV-2 could affect gonadal function,it is perhaps more common that generalized febrile illness may have a detrimental effect on spermatogenesis with a transient decrease in sperm concentration and/or quality.Indeed,this may be the more common mechanism to adversely affect spermatogenesis,given the lack of long-term impairment of semen parameters after COVID-19 that would be expected from significant orchitis.Furthermore,our patient was treated with clomiphene citrate that is associated with a temporary deterioration in semen quality for up to one in five men.Certainly,further studies are needed to elucidate the mechanisms by which SARS-CoV-2 might affect male fertility.
This case report suggests that COVID-19 may be associated with a quantitative defect in spermatogenesis.It remains to be determined whether there are clinical factors that correlate with the severity of impairment of spermatogenesis associated with COVID-19.It is possible that men with baseline defective sperm production may be at greater risk of effects from the febrile illness associated with COVID-19.Given the documented association between temporal decreases in sperm production and COVID-19,men presenting for infertility evaluation should be screened for COVID-19.
In summary,the long-term effects of COVID-19 on male fertility remain unknown.This report provides longitudinal documentation of a case of reversible cryptozoospermia after COVID-19.Further studies are needed to assess the mechanism and frequency of such infection and its effects on male fertility.
Author contributions
Study design:Spyridon P.Basourakos,Peter N.Schlegel.
Data acquisition: Spyridon P. Basourakos, Peter N.Schlegel.
Data analysis:Spyridon P.Basourakos,Peter N.Schlegel.
Drafting of manuscript:Spyridon P.Basourakos.
Critical revision of the manuscript:Spyridon P.Basourakos,Peter N.Schlegel.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2022年2期
Asian Journal of Urology2022年2期
- Asian Journal of Urology的其它文章
- Manuscript Guide for Authors
- Encrusted cystitis and ascites due to urethral calculus
- Clinical features and management of ureteric stump syndrome:Singlecentre experience and contemporary literature review
- Clinical lesson learned from genetic analysis in patients prior to surgical repair of hypospadias
- Factors influencing the degree of participation in surgical decision-making among Chinese patients with prostate cancer:A qualitative research
- Impact of delay from transperineal biopsy to radical prostatectomy upon objective measures of cancer control
