Zero thermal expansion in metal-organic framework with imidazole dicarboxylate ligands
Qilong Gao(高其龍) Yixin Jiao(焦怡馨) and Gang Li(李綱)
1Key Laboratory of Materials Physics of the Ministry of Education,School of Physics and Microelectronics,Zhengzhou University,Zhengzhou 450001,China
2College of Chemistry and Green Catalysis Center,Zhengzhou University,Zhengzhou 450001,China
Keywords: negative thermal expansion,metal-organic framework,zero thermal expansion,transverse thermal vibration,structure flexibility
1. Introduction
Most materials will expand when heated called as positive thermal expansion (PTE), one of which is attributed to the anharmonic lattice vibrations.[1]However, there also have very few compounds with abnormal thermal expansion,such as negative thermal expansion (NTE) or zero thermal expansion (ZTE) in a particular temperature zone.[2-4]Albeit rare,this phenomenon has increasing importance in thermal expansion controllable for highly sophisticated equipment and technology field such as telescopes and integrated circuit engineering.[5,6]So far, for the NTE mechanism,it is very complex. For example, phonon-related materials are found in many open-framework materials such as oxides,[7-10]fluorides,[13-17]and MOF.[18,19]Others are the electronic and magnetic transitions-related ones like chargetransfer[20,21](LaCu3Fe4O12and V2OPO4) and magnetovolume effect materials[22,23](ANMn3and Er2(Fe,Co)14B).Based on these NTE mechanisms, one can achieve the ZTE materials through chemical modification in single phase compounds, such as guest insertion,[24]nanometer effect,[25]and element substitution.[26]In fact,for the 1D or two-dimensional(2D)ZTE materials,it is also important to understand thermal expansion mechanism, while it has few reported. It is well known that the phonon-related mechanism has much larger NTE temperature range.[15,27]Hence,it is very worth exploring ZTE mechanism in the framework of material physics.
In recent years,metal-organic frameworks(MOFs)have attracted considerable interest, due to the unique pore structure and flexible framework, which focus on many applications such as catalysis, gas separation, and storage, sensing,drug delivery, energy, environment.[18,19,28]The net framework structure of MOFs is composed of metal ions or clusters through the bridging of organic ligands. Most MOFs have large flexibility,which depends on the presence of much weaker interactions (coordination bonds, hydrogen bonds orπ-πstacking).[18]There have reported many classical NTE materials in MOFs. For example, MOF-5 displays large isotropic NTE behavior,[19,29]other like Cu3BTC2,[30]UiO-66(Hf),[31]Cu-TDPAT,[32]and so on. The NTE-driven force in these MOFs is often attributed to transverse“skipping rope type”vibrations of the bridging organic ligands.[30-32]
In this work, we have found an interesting ZTE behavior in MOF-Sr ([Sr(DMPhH2IDC)2]n). The high-resolution variable-temperature powder x-ray diffraction (XRD) was conducted to investigate the structure and intrinsic thermal expansion and attempted ZTE mechanism from the the perspective of structural model.
2. Experimental methods
The sample of Sr-MOF has the same preparation method as reported in Ref. [33]. Thermal expansion behavior was characterized based on the high-resolution synchrotron XRD(SXRD), which was performed at the 11-BM-B beamline of Advanced Photon Source(λ=0.412634 °A).The lattice constants were extracted by using FULLPROF program[34]based on the Le Bail method. Thermal gravimetric analyses(TGA)were conducted on an NETZSCH STA 409PC synchronous thermal analyzer(heating rate of 10°C/min;in air).
3. Results and discussion
Recently, Xieet al.[34]reported one interesting substituted imidazoledicarboxylate based MOFs,[Sr(DMPhH2IDC)2]n[DMPhH3IDC=2-(3,4-dimethylphenyl)-1H-imidazole-4,5-dicarboxylic acid], possessing high proton conductivity (0.92×103S·cm1). Figure 1(a) shows the structure of Sr-MOF with the tetragonal phase (I41/a), of which constitutes with the Sr ion (eight-coordinated) and six H2DMPhIDC anions. To understand the framework better,one can use one atom to replace the organic ligands(Fig.1(b))and retain the Sr atoms to achieve one topological consideration of the three-dimensional (3D) network, as shown in Fig.1(c). It clearly observes that the structure like one honeycomb with 1D channels.
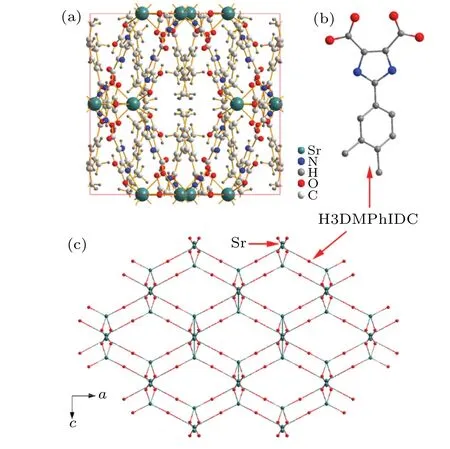
Fig.1. (a)Crystal structure of Sr-MOF([Sr(DMPhH2IDC)2]n). (b)Chemical structure of HDMPhIDC ligand, where the H atoms is omitted. (c) A structural unit diagram of Sr-MOF,where the ligands are simplified to one atom and the metal atom of Sr is retained.
In order to investigate the intrinsic thermal expansion of Sr-MOF, the variable temperature synchrotron x-ray diffraction was conducted from 100 K to 475 K at APS.Figure 2(a)shows the high resolution SXRD data of Sr-MOF with Le Bail fitted at 300 K.The fitted results indicated that the lattice parametera=16.79599(8) °A andc=17.57654(10) °A, which is consistent with the previous report.[33]Obviously,from the temperature dependence of the diffraction, the (100) diffraction peak shows essentially no shift from 100 K to 475 K,which indicates that the dimensions ofa-bplane is nearly ZTE as a function of temperatures. However, the(110)diffraction peak has much larger shift from 100 K to 475 K. In combination with the behavior of(100)diffraction peak,it suggests that the Sr-MOF has large thermal expansion alongcaxial direction. In order to exclude the factor of mass loss in the heating process for x-ray diffraction measurement, the TG-DSC measurement was conducted. As shown in Fig. 2(b), the results indicated that the Sr-MOF could keep stable up to 600 K.Hence,in the heating process of XRD measurement,no water molecules and ligands get out.

Fig.2. (a)The high resolution SXRD data of Sr-MOF with Le Bail fitted at 300 K.The inset shows the diffraction peak for(011)and(100)plane as a function of temperature. (b)The TG-DSC curve of Sr-MOF.
Here,the lattice constants were extracted with the LeBail pattern decomposition technique. The space group of structural model isI41/afrom 100 K to 475 K.Figure 3 shows the temperature-dependent lattice parameter change of Sr-MOF.The coefficient of thermal expansion(CTE)of the a axis displays near ZTE (average value-1.07(3) ppm/K) throughout the temperature range. While the CTE along thec-axis direction is quite large to +84.01(4) ppm/K. The volume CTE of Sr-MOF is calculated to be +81.97(3) ppm/K, which closes proximity to thecaxis. It is indicated that the material of Sr-MOF has large PTE alongcaxial and zero area thermal expansion behavior in thea-bplane.
It is well known that the MOFs as one kind of compounds have phonon-driven NTE behavior. Different from the traditional inorganic porous materials such as ZrW2O8[35]and ScF3,[36]whose NTE come from the transverse thermal vibration of O or F atoms. Here,the bridging parts are replaced to the polydentate ligands in MOFs,so the flexibility of polydentate ligands is the key for the thermal expansion. As shown in the structural unit diagram of Sr-MOF(Fig.1(c)), one can see that thea-bplane displays a square layer. Similar to the DABCO-based MOFs structure,[37]it exhibits near ZTE in their layered direction over a temperature range of 100 K-475 K.Due to the fact that the space steric ina-blayer is the largest,thus it could ensure the transverse thermal vibration of ligands. However, along thecaxis the angles of Sr-ligands-Sr linkage is much lower than 180°, it will cause the lengths of Sr-ligands-Sr linkage increasing with the temperature,thus lead to lager PTE in thecaxis.

Fig.3. (a)Temperature-dependent lattice parameter change of Sr-MOF,(b)the spatial distribution of CTE plotted by PASCal software.
4. Conclusion
In conclusion, we have investigated the intrinsic thermal expansion of Sr-MOFs through the high-resolution variabletemperature powder x-ray diffraction. We observed the ZTE behavior along thea-bplane and large PTE inc-axis direction. Such interesting results could attribute to the unique honeycomb structure, it could provide enough space for the transverse thermal vibration of polydentate ligands ina/b-axis direction, not for thec-axis direction. This work not only reports one 2D ZTE materials, but also provides some insight into exploring new abnormal thermal expansion materials.
Acknowledgments
Project supported by the National Natural Science Foundation of China(Grant Nos.22071221 and 21905252)and the Natural Science Foundation of Henan Province,China(Grant No.212300410086).
- Chinese Physics B的其它文章
- Helium bubble formation and evolution in NiMo-Y2O3 alloy under He ion irradiation
- Dynamics and intermittent stochastic stabilization of a rumor spreading model with guidance mechanism in heterogeneous network
- Spectroscopy and scattering matrices with nitrogen atom:Rydberg states and optical oscillator strengths
- Low-overhead fault-tolerant error correction scheme based on quantum stabilizer codes
- Transmembrane transport of multicomponent liposome-nanoparticles into giant vesicles
- Molecular dynamics simulations of A-DNA in bivalent metal ions salt solution

