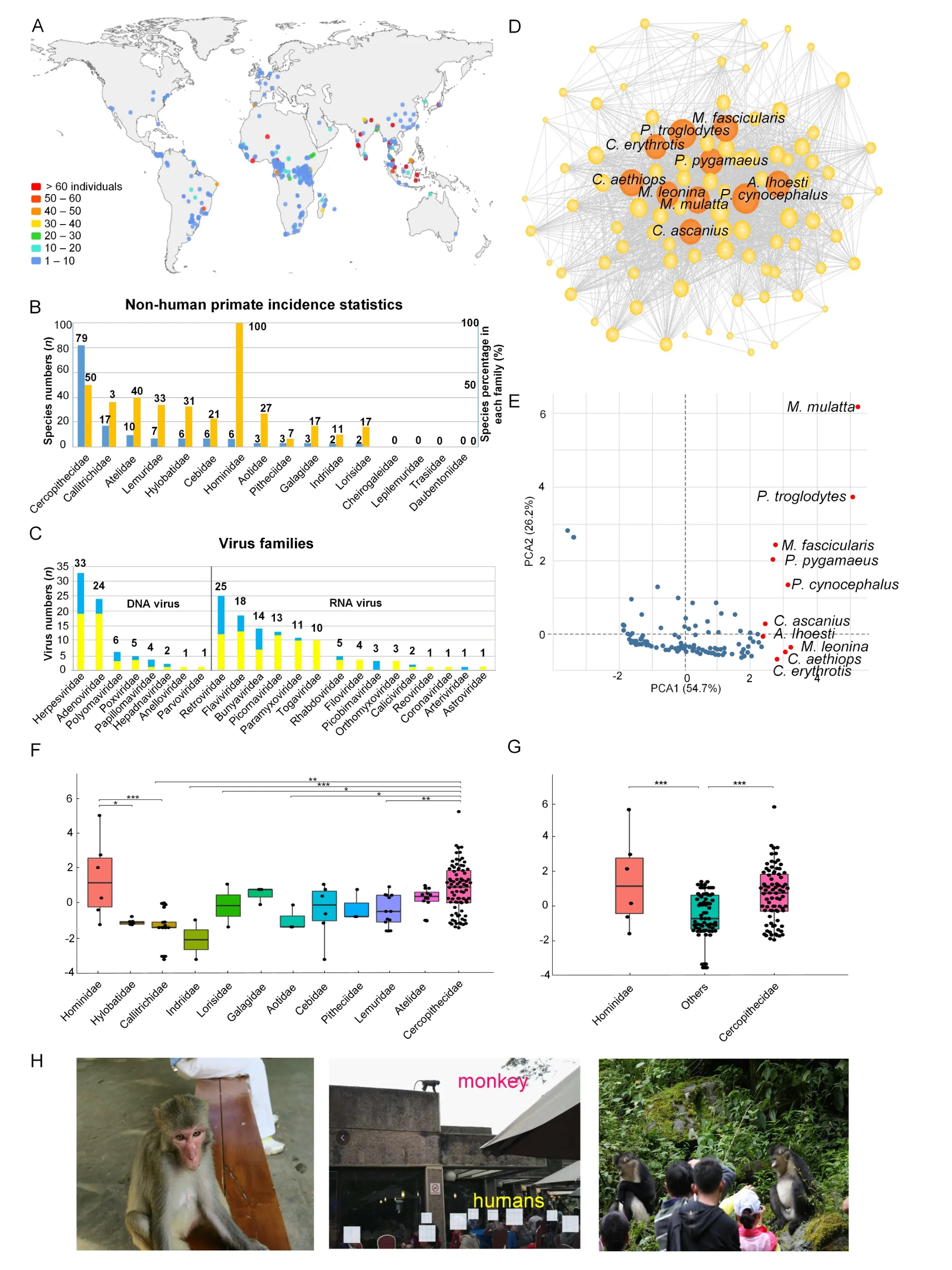
Global view on virus infection in non-human primates and implications for public health and wildlifeconservation
Viruses can be transmitted from animals to humans (and vice versa) and across animal species. As such, host-virus interactions and transmission have attracted considerable attention. Non-human primates (NHPs), our closest evolutionary relatives, are susceptible to human viruses and certain pathogens are known to circulate between humans and NHPs. Here, we generated global statistics on virus infections in NHPs (VI-NHPs) based on a literature search and public data mining. In total, 140 NHP species from 12 families are reported to be infected by 186 DNA and RNA virus species, 68.8% of which are also found in humans, indicating high potential for crossing species boundaries. The top 10 NHP species with high centrality in the NHP-virus network include two great apes (Pan troglodytes,Pongo pygmaeus)and eight Old World monkeys (Macaca mulatta,M.fascicularis,M. leonina,Papio cynocephalus,Cercopithecus ascanius,C. erythrotis,Chlorocebus aethiops, andAllochrocebus lhoesti). Given the wide distribution of Old World monkeys and their frequent contact with humans, there is a high risk of virus circulation between humans and such species. Thus, we suggest recurring epidemiological surveillance of NHPs, specifically Old World monkeys that are in frequent contact with humans, and other effective measures to prevent potential circulation and transmission of viruses.Avoidance of false positives and sampling bias should also be a focus in future work.
Emerging infectious diseases in humans can be caused by viruses derived from animals and several viruses are known to circulate between humans and animals. In the past few decades, cross-species transmission of viruses between animals and humans has been a major source of infectious disease and remains a global problem for public health and wildlife management (Zhou, 2020). For example, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread worldwide. Notably, recent studies suggest that pets and other animals may also be infected by SARS-CoV-2 through natural contact with humans (and vice versa) as well as across animal species (Tazerji et al., 2020; Helmy et al., 2020). As such,host-virus interactions and transmission are of great research interest (Shi et al., 2020).
From a compilation of 673 virus species and 415 mammalian and bird species, Mollentze & Streicker (2020)found that variation in the number of zoonoses in each mammalian order is consistent with a host-neutral model,whereby more species rich reservoir groups host more viruses and therefore a larger number of zoonotic species.Additionally, Johnson et al. (2020) found that wild mammals with declining populations due to exploitation and loss of habitat share more viruses with humans. Based on the associations between 1 785 virus species and 725 mammal species, Wells et al. (2020) showed that carnivores(Carnivora) and bats (Chiroptera) are central for the transmission of RNA viruses among mammalian groups, while ungulates (Artiodactyla and Perissodactyla) mainly transmit RNA and DNA viruses to other host species. It has also been reported that bats (Chiroptera), primates (Primates), and rodents (Rodentia) have a higher proportion of zoonotic viruses compared to other groups of mammals (Olival et al.,2017). These mammalian-scale studies have provided insights into virus transmission among various mammals and humans,thereby leading to a reconsideration of virus surveillance approaches.
Certain animal groups appear to be more frequently associated with zoonoses and the proportion of zoonotic viruses per host species can vary based on each mammalian order (Mollentze & Streicker, 2020). In rare cases, surveillance projects designed for all mammals in a certain geographical region are necessary. Furthermore, in addition to large-scale and class/order-level interpretation of the interactions between viruses and mammalian groups, effective virus surveillance and wildlife management strategies still require more detailed species-level information on each mammalian order (Devaux et al., 2019).
Among mammals, the close evolutionary relationship between humans and NHPs is believed to support pathogen transmission, with many viruses able to circulate between humans and NHPs (Devaux et al., 2019; Patrono et al., 2018).For instance, human coronavirus OC43 has been found in wild chimpanzees in C?te d′Ivoire (Patrono et al., 2018) and SARS-CoV-2 has been detected in captive gorillas following exposure to an infected but asymptomatic staff member at San Diego Zoo (https://www.the-scientist.com/news-opinion/us-confirms-worlds-first-sars-cov-2-cases-in-gorillas-68347).In addition, numerous viruses found in humans, including coronaviruses, enteroviruses, enteric adenoviruses,rotaviruses, and picobirnaviruses, have been detected in both captive and wild NHPs (Molina et al., 2019; Smith et al., 1982;Wang et al., 2007). Prominent cases of virus transmission from wild NHPs to humans include simian foamy virus (SFV),yellow fever virus (YFV), Zika virus (ZIKV), and human immunodeficiency virus (HIV) (Gómez et al., 2013; Nunn &Altizer, 2006; Wolfe et al., 2004, 2007). Conversely, several viruses found in NHPs, such as poliovirus and measles, are considered to be derived from local human populations (Wallis& Lee, 1999). Furthermore, organized feeding of NHPs is one of the most common forms of wildlife-related ecotourism,which increases the possibility of pathogen transmission(Devaux et al., 2019). Therefore, understanding the patterns of viral diversity in NHPs and determinants of cross-species transmission is essential to limit the potential circular transmission of viruses among humans and NHP species.
Here, we surveyed documented VI-NHPs based on database and literature searches. First, we generated summary statistics on reported VI-NHPs worldwide. We then constructed ecological networks of NHP hosts and their virus species. In these host-virus networks, nodes (hosts) linked by sharing virus species and nodes with higher centrality were considered better connected if they had more virus species in common. Consequently, the centrality of a particular node was a reflection of the number of virus species infecting the host and others and was deemed a good estimate of its potential as a source for harmful virus circulation (Gómez et al., 2013).In this way, we predicted NHP species with a high risk of virus transmission as well as geographic regions where virus circulation among humans and NHP species may be more likely to occur.
Based on 1 478 records extracted from the Global Mammal Parasite Database (GMPD, v2.0, http://www.mammalparasites.org/) and 43 VI-NHP reports (Supplementary Materials), we first generated an initial dataset describing the interactions between 123 NHP species and 141 virus species. Next, we consulted published reviews on VI-NHPs (Johnson et al.,2020; Mollentze & Streicker, 2020; Olival et al., 2017;Wachtman & Mansfield, 2012) to expand the dataset. The relationship between our initial dataset and collated dataset(from the four published reviews) is shown in Supplementary Figure S1. We finally obtained an integrated dataset containing 140 NHP species (12 families, 49 genera) infected by 186 virus species (Figure 1A-C). Only natural virus infections in captive and wild NHPs were selected, while artificial infections, e.g., virus inoculations for biomedical studies, were omitted. The viruses infecting NHPs covered nine DNA and 14 RNA virus families: i.e., Herpesviridae (33 viruses), Retroviridae (25), Adenoviridae (24), Flaviviridae(18), Bunyaviridae (14), Picornaviridae (13), Paramyxoviridae(11), Togaviridae (10), Polyomaviridae (6), Poxviridae (5),Rhabdoviridae (5), Filoviridae (4), Papillomaviridae (4),Orthomyxoviridae (3), Caliciviridae (2), Hepadnaviridae (2),Anelloviridae (1), Astroviridae (1), Picobirnaviridae (1),Parvoviridae (1), Reoviridae (1), Coronaviridae (1), and Arteriviridae (1) (Figure 1C). Among the 186 virus species reported in NHPs, 128 (68.8%) were shared with humans,indicating high zoonotic potential (Figure 1C).
The most documented VI-NHPs occurred in rhesus macaques (Macaca mulatta, 51 virus species; Supplementary Figure S2), 37 of which are shared with humans. The second most documented VI-NHPs were found in chimpanzees (Pan troglodytes, 40 virus species, Supplementary Figure S2).Notably, 94.4% of virus species identified in great apes(chimpanzees, bonobos, gorillas, and orangutans) are also reported in humans. Cercopithecidae (Old World monkeys)showed the highest number of species infected by viruses among all NHP families, with 79 infected species (56.4%) out of 159 Cercopithecidae species. Among other primate families, the number of species with known virus infection ranged from 0 to 17 (0.0%-100.0% of species members in each family, Figure 1B).
We then constructed a host-virus ecological network, with nodes representing NHPs linked through shared virus species.Centrality in the NHP-virus network can assess the potential for the circulation of viruses among NHPs and humans, thus we estimated centrality using four metrics, i.e., strength,eigenvector, betweenness, and closeness centralities, with the R package “igraph” (Lu et al., 2018) and UCINET v6.689(Borgatti et al., 2002). As each metric represents different and complementary aspects of centrality, we tested the correlations among all four indices. To clarify the effects of NHP species centrality on transmission ability, we obtained a“composite centrality” that integrates the different and complementary aspects of the four centrality metrics by principal component analysis (PCA) of the centrality index correlations (Cooper & Nunn, 2013). All four centrality indices showed positive correlations between each other(0.172 The phylogenetic generalized least squares (PGLS) method was used to test the relationship between centrality and the number of virus species reported in each NHP species, as well as the number of virus species found in both NHPs and humans (Nunn, 2011). After controlling for phylogeny, the number of virus species in each NHP species and the number of virus species shared with humans were significantly and positively associated with the centrality of each NHP species(strength, eigenvector, betweenness, closeness, and composite centralities; 0.331 Figure 1 Global view on virus infection in non-human primates (NHPs) and implications for public health and wildlife conservation Host-virus data can be sensitive to sampling effort.Consequently, the computation of individual centralities was largely influenced by the intensity of NHP species sampling.We addressed this problem by up-weighting the least sampled NHP species and down-weighting the most sampled NHP species per edge. For this, we corrected the weight of each edge by: WhereSVis the shared virus species,SP1 is the sampling effort of NHP species 1,SP2 is the sampling effort of NHP species 2, and sampling effort is the number of studies for each NHP species (Gómez et al., 2013). All four centrality indices showed positive correlations(0.344 We used the nonparametric Kruskal-Wallis test (Gómez et al., 2013) to assess whether the composite centrality measures differed among the NHP families. Furthermore, to account for sampling variation that could bias statistical results, the composite centralities of NHP families with few samples were merged. The composite centrality of Cercopithecidae was significantly higher than that of Callitrichidae, Indriidae, Lorisidae, Aotidae, and Lemuridae(P<0.05 in all cases, Figure 1F). However, no significant differences were detected between Cercopithecidae and the remaining six families (i.e., Hominidae, Hylobatidae,Galagidae, Cebidae, Pitheciidae, Atelidae).The composite centrality of Hominidae (humans not included) was significantly higher than that of Hylobatidae and Callitrichidae(P<0.05 in all cases, Figure 1F). Additionally, the composite centrality of Cercopithecidae and Hominidae was significantly higher than the merged data of the other NHP families(Figure 1G). Based on the assumption that geographic regions containing species more evolutionarily close to humans are more likely to be sources of zoonoses than regions containing fewer or more distantly related species, we hypothesized that the forests of central and western Africa represent regions where zoonotic outbreaks are likely to occur (Cooper et al.,2012; Pedersen & Davies, 2009). Our study supports this hypothesis and suggests that African Old World monkeys exhibit a high potential for the circulation of viruses with humans (Figure 1D). Interestingly, rhesus macaques from Asia, which are Old World monkeys, showed the highest reported virus infections in our study. Rhesus macaques are the world’s most widely distributed NHP species, occupying a vast geographic distribution spanning Afghanistan to the Chinese Pacific coastline and south into Myanmar, Thailand,Laos, and Vietnam (Zinner et al., 2013). Furthermore, longtailed macaques (M. fascicularis) are distributed over large parts of Southeast Asia and the Sundaland region between ca. N20?nd S10° (Zinner et al., 2013). The number of virus species detected in macaques is also reported to be significantly positively correlated with human contact frequency (Zhu, 2014). Our network analysis showed high centrality for the three macaque species in the NHP-virus network and ranked them among the top 10 most central NHPs. Consistently, 37 virus species were shared between humans and macaques (Supplementary Figure S2). Therefore, we call for comprehensive virus surveillance of NHPs to identify viruses with zoonotic potential. Moreover,programs investigating and monitoring the presence and transmission of viruses in captive and free-range NHPs,especially Old World monkeys and those in frequent contact with humans, are urgently needed to impede potential circulative virus transmission routes. For example,surveillance of howler monkeys in Brazil successfully identified a yellow fever virus outbreak in 2008-2009, which killed seven people and over 2 000 monkeys, thus prompting a successful large-scale human vaccination campaign (Agostini et al.,2014). In the past several decades, deadly viruses, such as rabies, herpes B, Marburg, and Ebola, have been transmitted from NHPs to humans. Zoonotic outbreaks are unusual but full of uncertainty and anxiety. For example, the epidemics of Ebola filovirus in 1977, AIDS/HIV in 1983, hantavirus in 1993,influenza A/H5N1 in 1997, Nipah virus in 1998, severe acute respiratory syndrome (SARS) coronavirus in 2003, and Middle East respiratory syndrome (MERS) coronavirus in 2012, were all of zoonotic origin (Devaux et al., 2019). It is also hypothesized that SARS-CoV-2 may be of zoonotic origin. If such investigations and precautions are neglected, SARSCoV-2 will not be the last animal-origin virus to be transmitted to humans. Viruses are also a threat to NHPs. The Zaire Ebola virus(ZEBOV) outbreak in humans is linked to a decrease in gorilla and chimpanzee populations in Gabon and Congo, and the same virus strain is estimated to have killed 5 000 gorillas in West Africa in 2002-2003 (Bermejo et al., 2006; Leroy et al.,2004). Experts in animal health and conservation have also called for the protection of great apes during the human COVID-19 pandemic, given that the transmission of human viruses to apes could result in severe outbreaks and extirpation of ape populations (Gillespie & Leendertz, 2020).Such efforts should also be expanded to Old World monkeys because: (1) our analysis showed that several Old World monkeys are at great risk of cross-species transmission due to their high centrality in the NHP-virus network; and (2)evolutionary genetic analysis has also predicted that Old World monkeys are more likely to be susceptible to SARSCoV-2 than other primates (Lu et al., 2020; Melin et al., 2020).Many Old World monkey species, particularly macaques,baboons, and green monkeys, live close to humans in rural and urban areas (Figure 1H), thus supporting virus transmission via direct contact. For example, human coronaviruses (HCoV) have been detected in a high number(22%) of baboons (Papio hamadryas) in Saudi Arabia(Olarinmoye et al., 2017). Macaques in India have been observed stealing testing samples of SARS-CoV-2-infected humans (https://www.theweek.in/news/india/2020/05/29/watch-monkey-steals-covid-19-patients-blood-samples-inmeerut.html). Furthermore, several Old World monkey species, especially macaques, are widely used animal models with large captive populations worldwide (Zhou, 2020). Old World monkeys living in natural reserves, parks, and temples are also frequently involved in ecotourism (Figure 1H; Afonso et al., 2021; Devaux et al., 2019). Thus, the possibility of cross-species virus transmission in great apes and Old World monkeys (and other NHPs) is extremely high, and further conservation efforts should be made to protect NHPs against virus transmission from humans and other animals. We note there are several limitations in host-virus network analyses based on database records and literature searches.First, compiling host-virus information from published literature may lead to “false positive” results. For example, there were seven virus species (herpesvirus SA8, simian retrovirus 2,simian T-lymphotropic virus, simian immunodeficiency virus,simian foamy virus, and measles) reported in indri (Indri indri)lemurs based on the GMPD records, but the original literature(Junge et al., 2011) mentioned that the tests for these viruses were negative. We have tried to minimize these kinds of errors in our study by checking the literature. However, there may be other issues that are difficult to capture. For instance, virus presence/absence in many species is based on serosurveys using serological assays targeting specific virus species. The efficiency of assays varies among host species, and the presence/absence of other virus species remains unknown. In addition, changes in the scientific naming of viruses and primate species could contribute to inconsistent species classifications. More detailed analysis should be handled by experts familiar with certain animal groups. Furthermore, host-virus interactions are sensitive to sampling effort. Even when controlling for general sampling bias, concentrated research on viruses in a specific NHP species may introduce bias. For example, intensive research activity on arboviruses supported by the Rockefeller Foundation in the first half of the 20th century, along with numerous virus discoveries in that field, introduced significant bias regarding NHP/virus interactions. Intensive research was also conducted on the origin of HIV in African NHPs,especially apes, with a comprehensive sampling effort in equatorial Africa (Patterson et al., 2019). Systematic pathogen surveillance based on virome screening using high-throughput sequencing can minimize sampling bias and error by using International Committee on Taxonomy of Viruses (ICTV)taxonomy and literature searches. The “One Health” approach emphasizes the connection between the health of humans, animals (wildlife and livestock),and the environment (Zinsstag et al., 2005). As bats, rodents,and birds are known to be natural reservoirs for many zoonoses, additional effort has been undertaken to understand the patterns of virus circulation between these species and humans. However, despite being the closest evolutionary group to humans, NHPs have been investigated somewhat rarely, although many virus species circulate between NHPs and humans. Here, we generated the latest and most comprehensive description of the interactions among virus species and NHPs, which included 140 NHP species and 186 virus species. Apes and Old World monkeys were shown to be the most central species in the NHP-virus network. Additionally, improving algorithms to avoid false negative/positive results in big data analyses, high-throughput sequencing to minimize sampling bias, and experts familiar with specific animal groups are highly recommended for future research. In summary, our NHP-virus network analysis shed light on host-virus interactions and highlighted those NHP groups with greater virus circulation potential, which should be the focus of future surveillance. The findings of our study should have implications for public health and wildlife conservation. SUPPLEMENTARY DATA Supplementary data to this article can be found online. COMPETING INTERESTS The authors declare that they have no competing interests. AUTHORS’ CONTRIBUTIONS Z.J.L., C.R., and M.L. conceived and designed the project.X.K.Q., M.H.H., J.L.Z., D.Y.L., T.H.W., Z.M.Y., L.Y.Z., Z.M.W.,H.J.N., K.Y.F., X.F.Z., M.M.C., and W.L.S. managed the project. Z.J.L., X.K.Q., M.H.H., and C.R. wrote and revised the manuscript. All authors read and approved the final version of t he manuscript. ACKNOWLEDGEMENTS The authors thank Fu-Wen Wei, Chung-I Wu, Wei-Wei Zhai,Qi Wu, Zheng-Long Wang, Hui-Jie Qiao, Paul Garber, and Martin Burrows for data analyses and English corrections.