基于吡啶-2,6-二羧酸構(gòu)建的鐠バ配合物的合成、表征和生物活性
張 莉 郭林峰 黃 勝 康 杰 孫偉明
(福建醫(yī)科大學(xué)藥學(xué)院,福建省藥物靶點(diǎn)發(fā)現(xiàn)與結(jié)構(gòu)功能研究重點(diǎn)實(shí)驗(yàn)室,福州 350108)
0 Introduction
Nowadays, one of the most rapidly developing areas of pharmaceutical research is the discovery of novel drugs for the cancer therapy. As is well known,thecis-diamminedichloroplatinumギ(cisplatin) continues to be one of the most effective antitumor drugs available for therapeutic of solid tumors, such as germ cell tumors,ovarian,lung,head and neck,bladder cancers until now[1-3]. Despite the great utility of cisplatin,its therapeutic value is also accompanied by serious side effects, such as nephrotoxicity, neurotoxicity,emetogensise, and ototoxicity[4-5]. To overcome these disadvantages, more and more compounds have been synthesized and investigated in the past decades.Among them, transition metal complexes have been proved to be capable of effectively restraining the reproduction of cancer cells, and therefore, some of such compounds have been successfully used in clinical treatments[6]. These successful cases have stimulated more research interests to explore new antitumor agents in the area of complexes.
During the past few decades, the coordination chemistry has attracted great attention because of the intriguing topological structures and potential applications of complexes in gas storage and separation, catalysis, magnetism, sensing and cancer therapy[7-16]. In contrast to transition metal complexes, the chemistry of lanthanide - based complexes is far less developed though the lanthanide metal ions possess many interesting inherent properties[17]. This may be partially attributed to the fact that the coordination of the lanthanide metal ions is less predictable as compared to the other transition metal counterparts. The lanthanide metal ions existing mainly in their trivalent state have variable coordination numbers and flexible coordination environments.Besides,the well-known lanthanide contraction can provide structural diversity, and therefore lead to different properties upon the subtle alteration in the synthesis[17-21]. However, in the view of crystal engineering, the design of lanthanide-based complexes is still challenging.
It is well-known that the trivalent lanthanide ions have a large radius and high affinity for oxygen donor atoms, thus, multi - dentate ligands with oxygen or hybrid oxygen-nitrogen atoms,such as pyridine carboxylate, imidazole dicarboxylate and thiophene dicarboxylate have been employed in the fabrication of novel lanthanide-based complexes[22]. Among various ligands,pyridine-2,6-dicarboxylic acid (H2pdc), a very important carboxylate derivative, has attracted much interest in coordination chemistry. Because it has a rigid angle of 120° between the central pyridine ring and two carboxylate groups, and therefore could potentially provide various coordination modes to form both discrete and consecutive metal complexes under appropriate synthesis condition. According to a survey on the lanthanide-H2pdc complexes submitted to the Cambridge Structure Database[23], H2pdc ligand almost adopts the fully deprotonated form in the resulting complexes.Based on this ligand, a large number of beautiful lanthanide-based complexes of ingenious design have been constructed up to now[18,22,24-26].
To explore more complexes with antitumor activity,a novel lanthanide-based complexes (H2pipz)1.5[Pr(pdc)3]·7H2O (1, H2pipz=biprotonated piperazine) has been synthesized by using H2pdc as ligand in the presence of piperazine under hydrothermal condition in this work. Complex 1 was firstly characterized by IR, elemental analysis, single-crystal X-ray diffraction analysis, and theoretical study. Afterwards, the inhibition of complex 1 against chronic myelocytic leukemia (K562)and esophageal carcinoma (OE-19) cells was determined by using microculture tetrazalium (3-(4, 5-dimethylthiazo l-2-yl)-2,5-diphenyltetrazolium bromide,MTT)assay.Our results revealed that complex 1 exhibited certain antiproliferation effects on both K562 and OE-19 cells with IC50values of (61.3±10.2) μg·mL-1and(15.9±3.2)μg·mL-1,respectively.
1 Experimental and theoretical methods
1.1 Materials and physical measurements
All chemicals were purchased commercially and used without further purification. K562 and OE-19 cells were obtained from Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Infrared spectrum was recorded in a range of 400~4 000 cm-1on a Perkin-Elmer Spectrum-2000 FT-IR spectrometer using KBr pellets.Elemental analysis (C,H and N)was carried out with a Perkin-Elmer 240 elemental analyzer.
1.2 Synthesis
A mixture of Pr(NO3)3·6H2O (1.2 mmol, 0.522 g),H2pdc (2.05 mmol, 0.342 g), piperazine (1.2 mmol,0.103 g)and H2O(10 mL)was sealed in a 25 mL Teflon-lined autoclave at 120 ℃for 5 d and then cooled to room temperature. Then, after suction filtration and being washed by water, transparent green prismatic crystal of complex 1 was obtained (Yield: 70% based on Pr). Anal. Calcd. for C54H76Pr2N12O38(%): C, 38.67;H, 3.97; N, 10.02. Found(%): C, 38.62; H, 4.02; N,9.98.IR spectrum (KBr pellet,cm-1):3 450~3 019(br),1 569(vs),1 394(s),1 018(m),769(m),661(w).
1.3 Crystal structural determination
A suitable single crystal with dimension of 0.33 mm×0.13 mm×0.12 mm of complex 1 was selected for X-ray analysis.The diffraction data were collected on a Rigaku Saturn 724 CCD diffractometer equipped with a graphite-monochromated MoKαradiation (λ=0.071 073 nm) using aφ-ωscan mode at 293(2) K.Absorption corrections were applied using multi-scan after the data reduction was performed using Crystalclear program[27]. The structure was solved by direct methods and refined by full-matrix least-squares onF2by using SHELXS-97[28]and SHELXL-2014/7[29]programs, respectively. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were located geometrically and treated as riding atoms with a common fixed isotropic thermal parameter. The crystallographic data are collected in Table 1. Meanwhile, the selected bond distances and angles of complex 1 are given in Table 2, and the H-bonding parameters are summarized in Table 3.
CCDC:1834053.
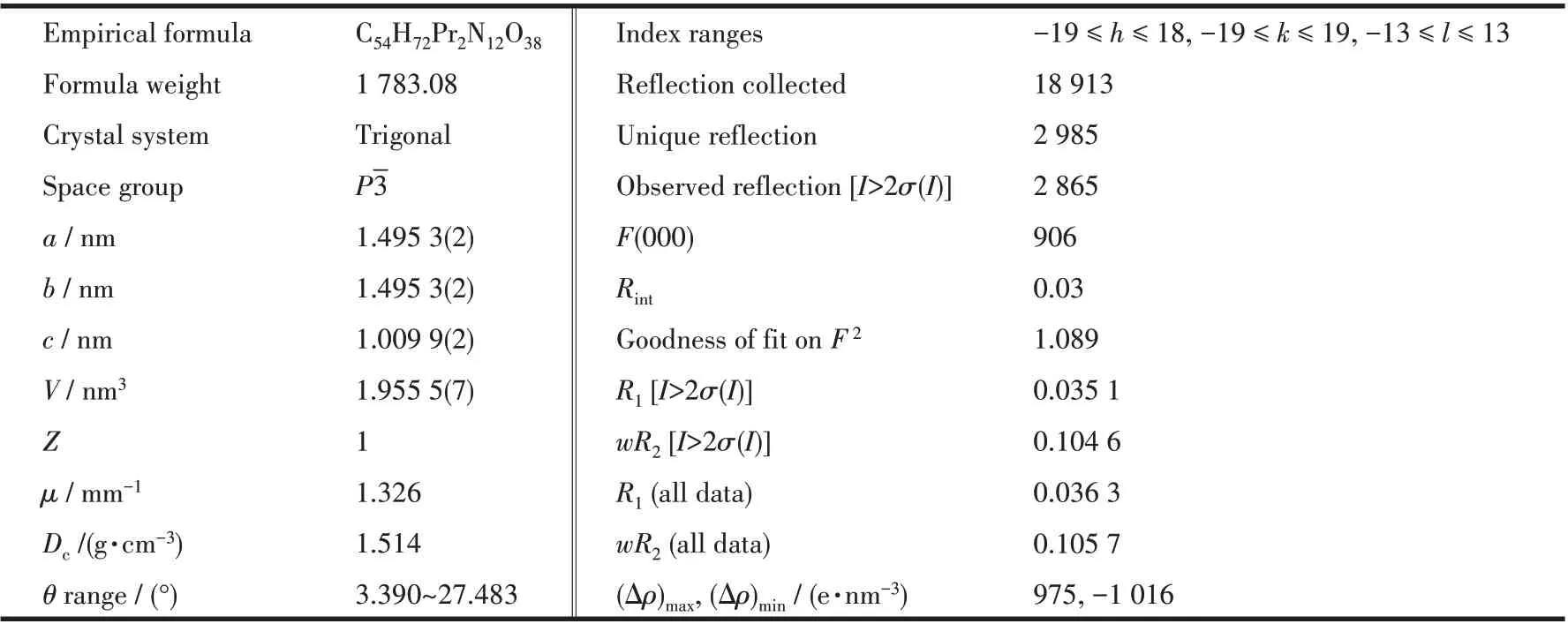
Table 1 Crystallographic data collection and structure refinement details
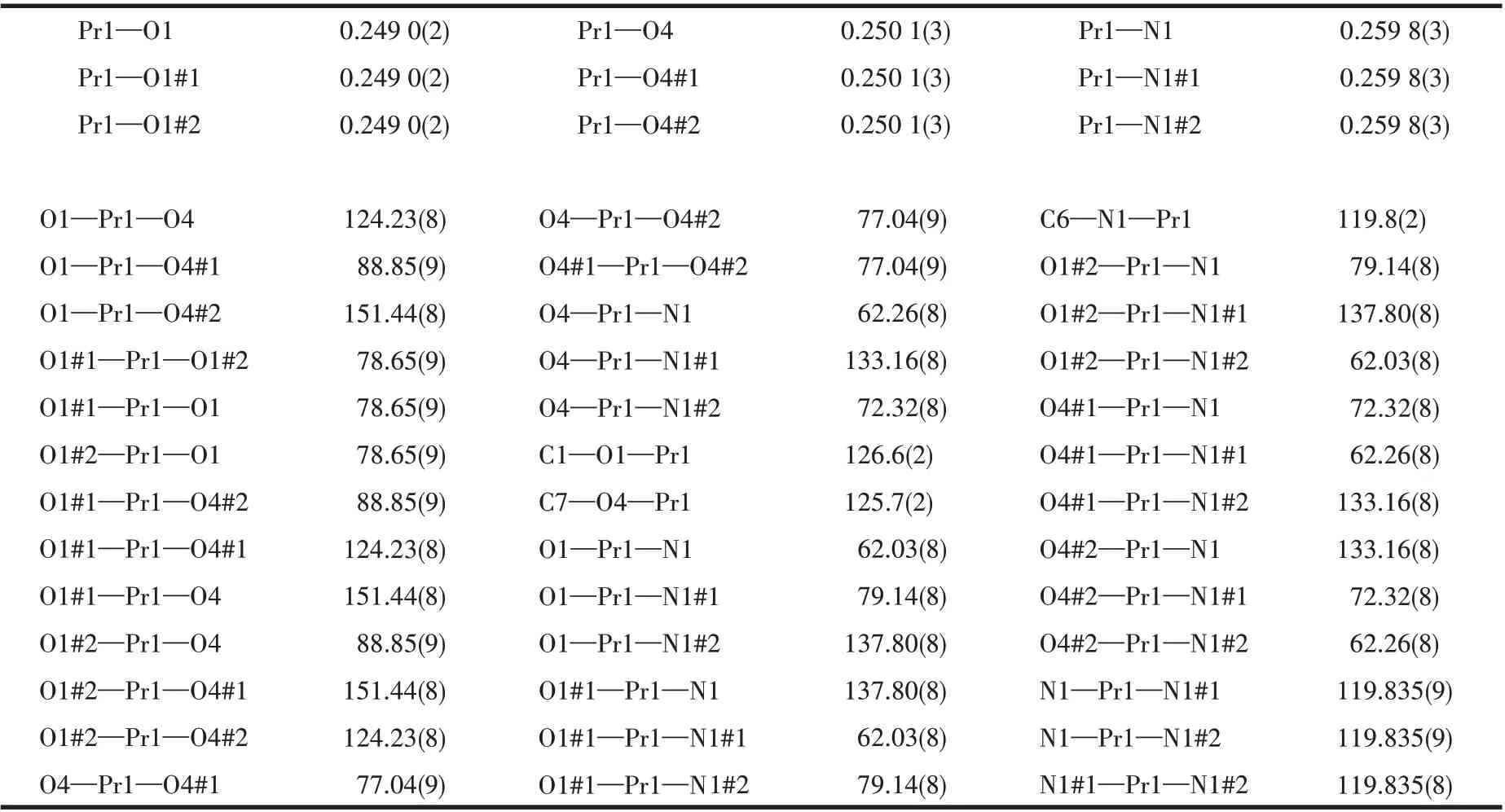
Table 2 Selected bond lengths(nm)and bond angles(°)for 1
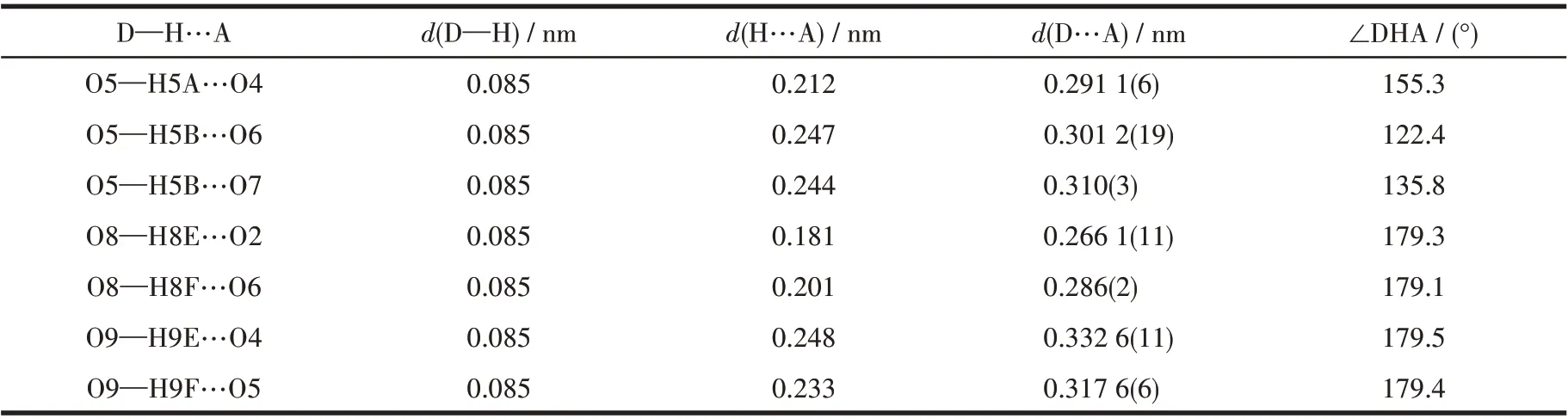
Table 3 Hydrogen parameters for complex 1
1.4 Cytotoxicity in vitro
The proliferation inhibition activities of this studied complex against K562 and OE19 cells were assessed by MTT assay. Briefly, the K562 and OE19 cells were cultured in RPMI-1640 medium supplemented with 0.25% trypsin and grown at 37 ℃in a humidified atmosphere in the presence of CO2(Volume fraction: 5%). Harvested cells were diluted to 1×105mL-1and then seeded into a 96-well culture plate with 100 μL per well, respectively. After incubation at 37 ℃in a 5% CO2incubator for 24 h, the samples containing the tested complex solutions of serial concentrations were added into the wells of the experimental groups(10 μL per well). Meanwhile, the dimethylsulfoxide(DMSO) without complex 1 was added into the well of the control group (10 μL per well). The cells were sequentially incubated for 72 h, followed by the addition of 20 μL MTT solution (2 mg·mL-1in D-Hanks)to each well and further cultivation for 4 h. Afterwards,the supernate with MTT was removed and followed by the addition of 150 μL DMSO to dissolve formazan dye for 10 min at room temperature along with slow oscillation. Finally, the optical density (OD) at 490 nm was read by an enzyme - linked immunosorbent assay(ELISA)reader and the inhibition rate,IR=(1-ODcomplex/ODblank)×100%, were calculated. Herein, the samples containing complex 1 were obtained by dissolving the isolated crystal into DMSO following a high temperature sterilization.Thereafter,these solutions were diluted by RPMI 1640 nutrient solution to the concentrations of 100, 50, 25, 12.5, 6.25, 3.13, 1.56, 0.78 μg·mL-1.
1.5 Computational details
The geometric structure of the subunit [Pr(pdc)3]3-of complex 1 was optimized at B3LYP/6-31++G**&SDD level, where 6-31++G** basis set was employed to describe hydrogen, carbon, oxygen and nitrogen,and the relativistic effective core potential of Stuttgart/Dresden (SDD)[30]was used for Pr. The electrostatic potential (ESP) and frontier molecular orbitals(FMO) of complex 1 were analyzed based on the optimized structure using GaussView program[31]. The changes in enthalpy and Gibbs free energy for considered dissociation reactions were computed under 298 K and 101.325 kPa at the same level.All the above calculations were carried out by using a Gaussian 16 program package[32], in which self-consistent reaction field(SCRF) calculations with polarizable continuum model(PCM)[33]were performed to consider the solvent effects of water.
2 Results and discussion
2.1 Synthesis and characterization
Complex 1 was obtained under hydrothermal conditions at 120 ℃and appeared as green transparent crystals. Herein, the IR spectrum of complex 1 is shown in Fig.1. From Fig.1, it was observed that the strong and broad absorption bands were in a range of 3 000~3 700 cm-1, which are attributed to the O—H stretching vibrations of the coordinated and crystal water molecules. The absence of strong absorption bands around 1 700 cm-1demonstrates that all of the carboxyl groups of the ligands are deproponated and coordinated to the Pr3+ions. The strong vibrations appearing around 1 600 and 1 400 cm-1correspond to the asymmetric and symmetric stretching vibrations of the carboxylate group, respectively. The absorption bands in the ranges of 660~670 cm-1and 720~770 cm-1in the fingerprint region are attributed to Pr—O and P—N vibrations,respectively.
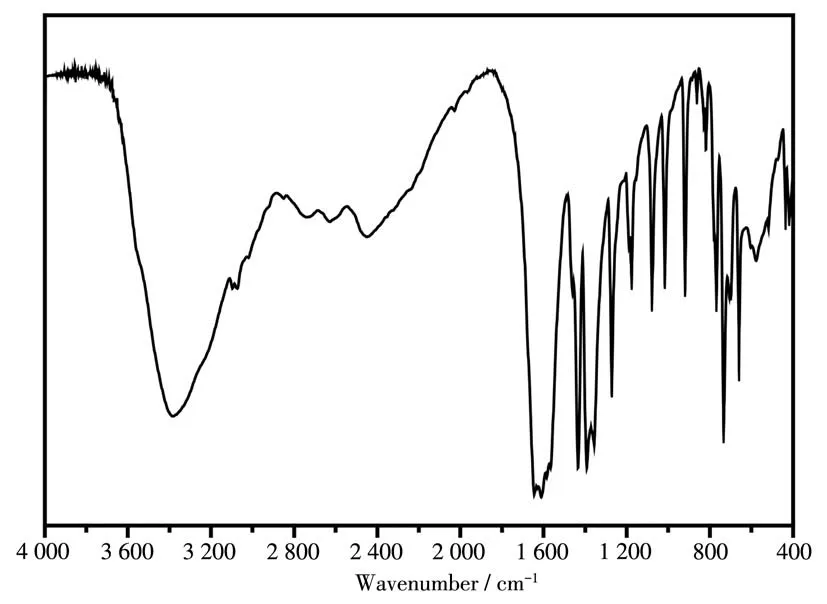
Fig.1 IR spectrum of complex 1
2.2 Crystal structure
As shown in Table 1, the structure of 1 belongs to the triclinic system withP3 space group. From Fig.2a,it is noted that its molecular structure contains a mononuclear [Pr(pdc)3]3-unit. As shown in Fig. 2b, the[Pr(pdc)3]3-monomers form a 2D layer alongaandbaxes through theπ-πstacking between pdc2-ligands.Then,the interlayer space is filled with a layer of piperazine molecules, which serve as an auxiliary supporting bridge to connect two adjacent 2D layers of[Pr(pdc)3]3-units to form the 3D supramolecular structure of complex.
From Fig.2a, it can be seen clearly that the Pr3+ion in complex 1 is nine-coordinated: six carboxylate oxygen atoms (OCOO—) and three nitrogen atoms from pdc2-ligands. It is found that these nine coordinated atoms form a distorted tri-capped triangle prism structure with the nitrogen atom occupying the capped apex,which was also observed in previously reported lanthanide complexes,such as the La,Tb,and Ce-based complexes[34-35]. Herein, the average lengths of Pr—N and Pr—O bonds are 0.259 8 and 0.249 6 nm,respectively,which are consistent with those of reported Pr—N(0.259 4 nm) and Pr—O (0.250 5 nm)[36]. Moreover, it is observed that the coordination mode adopted by three pdc2-ligands are identical in each subunit,namely, every 2,6-pdc2-dianion is coordinated to Pr3+by the tridentate chelation mode. It should be mentioned that,during our submission, a Pr-based complex with the same ligands but different space group and crystal parameters was reported by Moghzi et al[37]. This reported complex also has the similar coordination environment and nearly equal Pr—N (0.261 9 nm) and Pr—O(0.249 8 nm) bond lengths to complex 1 in this work.However, it is worth noting that all the above-mentioned lanthanide complexes are only tested to show good luminescent behavior[34-35]or the ability to adsorb iodine[37]. Differently, to explore the potential of these zero-dimensional lanthanide complexes in antitumor application, the cytotoxicityin vitroof complex 1 was evaluated in this work.
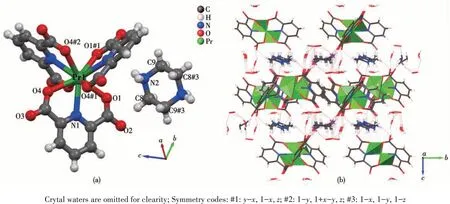
Fig.2 Molecular structure showing the atom numbering scheme(a)and 3D cell space-filling structure(b)of 1
2.3 Cytotoxicity in vitro
Now,turn to the key question of the study:can the synthesized complex 1 exhibit antitumor activity? To answer this question, thein vitrogrowth inhibitory effect of complex 1 on two kinds of human tumor cells,i.e., K562 and OE-19 cells, was evaluated by MTT assay in this work. After a period of 72 h treatment, it was found that complex 1 possessed certain antitumor activity upon K562 and OE-19 cells. The inhibition rates of complex 1 on K562 and OE-19 cells under different concentrations are showed in Fig. 3. From Fig.3, it is observed that the inhibition rates on K562 and OE-19 cells gradually increased along with the increasing concentrations. In particular, the obtained IC50values for K562 and OE-19 cells were (61.3±10.2)μg·mL-1and (15.9±3.24) μg·mL-1, respectively. This indicates that the synthesized complex appears to be more cytotoxic for OE-19 cells as compared to K562 cells, and thus it exhibits a great potential to serve as an effective agent for restraining the growth of OE-19 cells. Though complex 1 only exhibited a bit higher IC50value than cisplatin ((5.89±1.12) μg·mL-1)[38]on OE19 cells, it is still highly expected that complex 1 could be further tested to serve as antitumor agent because it may avoid the serious neurotoxicity, cytotoxicity and nephrotoxicity[4-5]accompanied by the utilization of cisplatin.
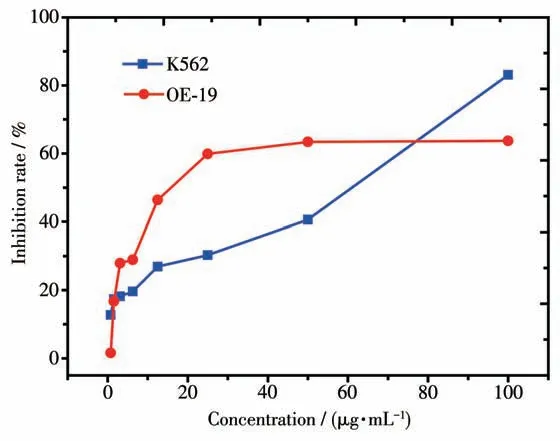
Fig.3 Inhibition rates of 1 against K562 and OE-19 tumor cells with different concentrations of 1
2.4 Quantum chemistry calculations
Considering the antitumor activity of 1 upon K562 and OE - 19 cells, the electronic structure of its[Pr(pdc)3]3-subunit was also analyzed by density functional theory (DFT) calculations in this work. Herein,the piperazine molecules were neglected because these auxiliary bridging molecules had little effect on the electronic properties of[Pr(pdc)3]3-units after being dissolved in solution. The ESP and FMO of [Pr(pdc)3]3-in aqueous solution are illustrated in Fig.4.
It is known that the ESP maps are often used to identify sites with negative and positive electrostatic potentials for electrophilic attack and nucleophilic reactions. As shown in Fig.4a, the central region of[Pr(pdc)3]3-shows the largest negative potential and thus is suitable for electrophilic attack, whereas the positive regions mainly focus on peripheral hydrogen atoms of pdc2-ligands. Besides, it is observed from Fig.4b and 4c that the HOMO is mainly composed of the 4fatomic orbital of central Pr, while the large proportion of electron cloud of LUMO is distributed on the three surrounding pdc2-ligands. From these analyses,it is hoped that these surrounding ligands have a large tendency to bind with the electronegative nucleic acid bases of DNA viaπ-πstacking interactions when it approaches DNA in cells, which can be regarded as a possible reason for the antitumor activity of complex 1.Besides, the large HOMO-LUMO gap of 3.10 eV for[Pr(pdc)3]3-indicates that this [Pr(pdc)3]3-monomer has high chemical stability in aqueous solution. Moreover,in order to explore whether the [Pr(pdc)3]3-unit can be dissociated in water solution, the dissociation reactions of complex 1 in aqueous solution have been also considered. Our DFT calculations reveal that the Gibbs free energy change (ΔG) and enthalpy change (ΔH) for[Pr(pdc)3]3-→Pr3++3pdc2-are as large as 476.18 and 630.78 kJ·mol-1, respectively at 298 K and 101.325 kPa.This demonstrates that the Pr3+and pdc2-are tightly bound together in complex 1, resulting in its high thermodynamic stability in solution.
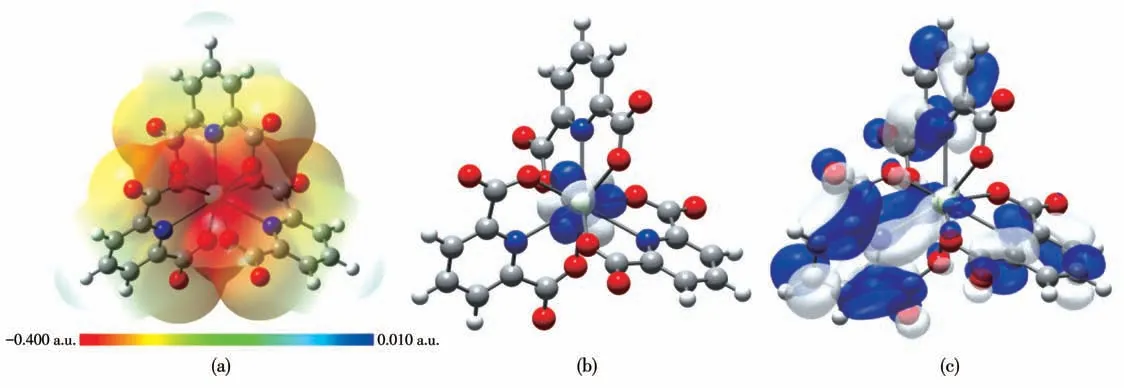
Fig.4 ESP and FMO of[Pr(pdc)3]3-of complex 1:(a)ESP,(b)HOMO,(c)LUMO
3 Conclusions
In this work, a novel lanthanide-based complex,i.e., (H2pipz)1.5[Pr(pdc)3]·7H2O (1, pdc=pyridine-2,6-dicarboxylate and H2pipz=biprotonated piperazine),was synthesized under hydrothermal condition and characterized by IR spectrum, elemental analysis, and X-ray single-crystal diffraction. The electronic structure of complex 1 was studied by means of DFT calculations to investigate the ESP and FMO of its subunit[Pr(pdc)3]3-in water. Moreover, a significant antitumor activity of complex 1 against K562 and OE-19 cells was experimentally verified by MTT assay, which demonstrates its great potential of serving as a new antitumor agent.
 無(wú)機(jī)化學(xué)學(xué)報(bào)2021年7期
無(wú)機(jī)化學(xué)學(xué)報(bào)2021年7期
- 無(wú)機(jī)化學(xué)學(xué)報(bào)的其它文章
- 大碳層間距的瀝青基多級(jí)孔碳材料的制備及其在超級(jí)電容器中的應(yīng)用
- Mo摻雜調(diào)控電子結(jié)構(gòu)增強(qiáng)NiS電催化固氮性能
- 剛性配體經(jīng)自發(fā)拆分構(gòu)筑的dia型螺旋對(duì)映框架
- 功能化金屬有機(jī)框架材料催化二氧化碳轉(zhuǎn)化研究進(jìn)展
- 溶劑熱一鍋法合成Ag-TiO2微球及其對(duì)過(guò)氧化氫的電化學(xué)檢測(cè)
- 通過(guò)能量傳遞實(shí)現(xiàn)Ba5-2x-yTbxNax(PO4)3Cl∶yEu2+熒光粉的光譜調(diào)控
