Dielectric Phase Transition Induced by Halogen Substitution Based on 1,4-Diazabicyclo[2.2.2]octane-Derivatives
CHENG Sai-NanDING KunSONG Shuang-Teng ZHANG Tie ZHANG Zhi-Xu ZHANG Yi FU Da-Wei
(Ordered Matter Science Research Center,Jiangsu Key Laboratory for Science and Applications of Molecular Ferroelectrics,Southeast University,Nanjing 211189,China)
Abstract:We successfully synthesized(X-EtHDabco)[ZnBr4](X=H,F,Cl for compound 1,2,and 3,EtDabco=N-ethyl-1,4-diazoniabicyclo[2.2.2]octane)through precise molecular design.Systematic measurements(powder X-ray diffraction,differential scanning calorimetry measurements,dielectric measurements)were performed on these compounds.It is noteworthy that,no hint of heat anomaly was observed for 1,while both 2 and 3 displayed intriguing isostructural order-disorder phase transition near 230 K.Through the analysis of the crystal structure,the orderdisorder transformation of organic ammonium plays a major role in the phase transition,in addition,the displacement of the inorganic framework also contributes to dielectric response.CCDC:2032032,1(293 K);2032034,2(213 K);2032033,2(293 K);2031906,3(298 K);2032037,3(202 K).
Keywords:halogen substitution;organic-inorganic hybrid;reversible phase transition;dielectric properties;single crystal
Over the past few decades,phase transition materials have attracted tremendous interests due to their potential applications in information storage,electronic devices,sensing,etc[1-6].These responsive materials can switch between the high and low dielectric states under the stimulation of temperature,light,and pressure.Previous research concentrated on inorganic ceramics,however,high cost and complex synthesis process hin-der its development[7-13].Accordingly,enormous studies have devoted to organic-inorganic hybrid perovskites(HOIPs),which benefit from low cost and environmentally friendliness by introducing flexible organic cations[14-17].Nonetheless,the realization of excellent physical properties is still challenging[17-20].
Among HOIPs,the compounds with flexible moieties have been supposed as excellent candidates to design new phase transition materials[21-26].More precisely,Dabco(1,4-diazabicyclo[2.2.2]octane),as a highly symmetric diamine,has been widely used to construct dielectric materials even ferroelectrics[27-28].Reportedly,(MeHDabco)RbI3achieved ferroelectric at 430 K through the introduction of methyl towards the Dabco[29].Moreover, the successful synthesis of[(CH3)3NCH2X]PbI3(X=H,F,Cl,Br,and I)demonstrated that the introduction of halogen atoms is an efficient strategy in designing molecular ferroelectrics[30-35].The combination of organic cations with halogen atoms plays a decisive role in affecting the structural characteristics[36-40].The compounds[Cu(DabcoCH2Cl)(H2O)Cl3][41],[Cu(DabcoCH2Cl)(H2O)Br2.75Cl0.25][42],[Mn(DabcoCH2Cl)(H2O)Cl3][43]and[Cu(DabcoCH2Br)(H2O)Br3][44]were successfully synthesized,which proved that the confined environments of Dabco moieties can be tuned by the metal ions or the N-halomethyl substituents.
Inspired by these successful molecular designs,we report a class of zero-dimensional materials:(H-EtHDabco)[ZnBr4](1),(F-EtHDabco)[ZnBr4](2)and(Cl-EtHDabco)[ZnBr4](3)(EtDabco=N-ethyl-1,4-diazoniabicyclo[2.2.2]octane).It is noteworthy that compounds 2 and 3 displayed reversible structural phase transition around 230 K by introducing a halogen atom(F or Cl),whereas no hint of phase transition was observed for 1.The differences in phase transition behavior suggest that slight changes in the organic cations can affect the overall structural characteristics.Furthermore,the order-disorder transformation of organic ammonium plays a major role in the phase transition,besides,the displacement of the inorganic framework also contributes to dielectric response.In the meantime,approximate van der Waals radii(F:0.064 nm,Cl:0.099 nm)and analogous intermolecular interaction are the main cause of the similarity in structure and properties between 2 and 3.
1 Experimental
1.1 Sample preparation
All analytical-grade chemicals were obtained from commercial sources and used without further purification.(H-EtHDabco)2+,(F-EtHDabco)2+and(Cl-EtHDabco)2+were synthesized according to the reported method[45],as shown in Scheme 1a.(X-EtHDabco)Br2(X=H,F,Cl)and ZnBr2were stirring to dissolve in hydrobromic acid solution(Scheme 1b).Colorless bulk crystals were obtained by evaporation at room temperature for several days.The phase purity of the compounds was determined according to powder X-ray diffraction(PXRD).
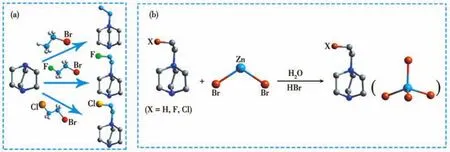
Scheme 1 Synthesis of(X-EtHDabco)[ZnBr4],in which X=H,F,Cl for compounds 1,2 and 3,respectively
1.2 Single crystal X-ray crystallography
Variable-temperature single-crystal X-ray diffraction data were collected at 293 K,213/293 K and 202/298 K for 1,2 and 3.The Crystal Clear software pack-age was utilized for data collection,cell refinement and data reduction.The crystal structures were solved by direct methods and refined with a full-matrix leastsquares method based onF2data with the SHELXTL software package[46].All non-hydrogen atoms were refined anisotropically using all reflections withUiso=1.2Ueq,and the positions of the hydrogen atoms were added geometrically and refined using the“riding”model withUiso=1.2Ueq.Details about the data collection and refinement of compounds 1,2 and 3 were summarized in Table S1~S3 (Supporting information),respectively.
1.3 Thermal measurements and infrared spectroscopy
Differential scanning calorimetry(DSC)measurements of the compounds were performed on a NETZSCH DSC 200F3 instrument under nitrogen atmosphere.Compounds 1(5.7 mg),2(23.6 mg)and 3(28.8 mg)were placed in aluminum crucibles.Then the samples were heated and cooled at the rate of 20 K·min-1in a temperature range of 173~303 K or 173~333 K,respectively.In addition,the IR spectra of three compounds were collected on a Nicolet 5700 spectrometer at room temperature.Thermogravimetric(TG)analyses were performed on a TA Q50 system with a heating rate of 10 K·min-1under a nitrogen atmosphere.
1.4 Dielectric measurements
The temperature-dependent dielectric measurements were performed on the Tonghui TH2828A instrument in a temperature range of 173~273 K(2)or 173~333 K(3),respectively,in the frequency range from 500Hz to 1MHz under the measuring AC voltage of 1V.
2 Results and discussion
2.1 DSC,IR and PXRD analysis
The phase purities of the compounds were confirmed by PXRD(Fig.S1)and IR analysis(Fig.S2).Infrared characteristic peaks of the C—F bond from(F-EtHDabco)2+cation and the C—Cl bond from(Cl-EtHDabco)2+cation,were clearly shown at around 1 090 and 806 cm-1,respectively.
To investigate the phase-transition behavior,DSC measurements were applied on the polycrystalline samples of 1,2 and 3.As shown in Fig.1a,no hint of thermal anomaly was observed for 1.When the H atom of(H-EtHDabco)2+cation was replaced by a fluorine or chlorine atom,a pair of endothermal and exothermal peaks were detected at 228.75/215.95 K and 228.45/215.45 K of 2,3,respectively.Remarkably,the sharp peaks and the large thermal hysteresis of 12.8 and 13 K reveal their first-order type phase transition characteristics(Fig.1b,1c).Based on the DSC curves,the average entropy changes ΔSwere estimated to be 21.67 and 3.37 J·mol-1·K-1for 2 and 3,respectively.According to the Boltzmann equation ΔS=RlnN(Rrefers to the molar gas constant),the values ofNwere calculated to be 2.6 and 1.50 for 2 and 3,respectively.The configuration value ofNimplies the order-disorder transformation of the molecules during the phase transition.As illustrated in Fig.S3,1,2,and 3 showed thermal stability at 514,580 and 558 K,respectively.Such excellent stability may benefit potential device applications.For convenience,we marked the phase belowTtr(transition temperature)as LTP (low-temperature phase),the phase aboveTtras RTP(room temperature phase).
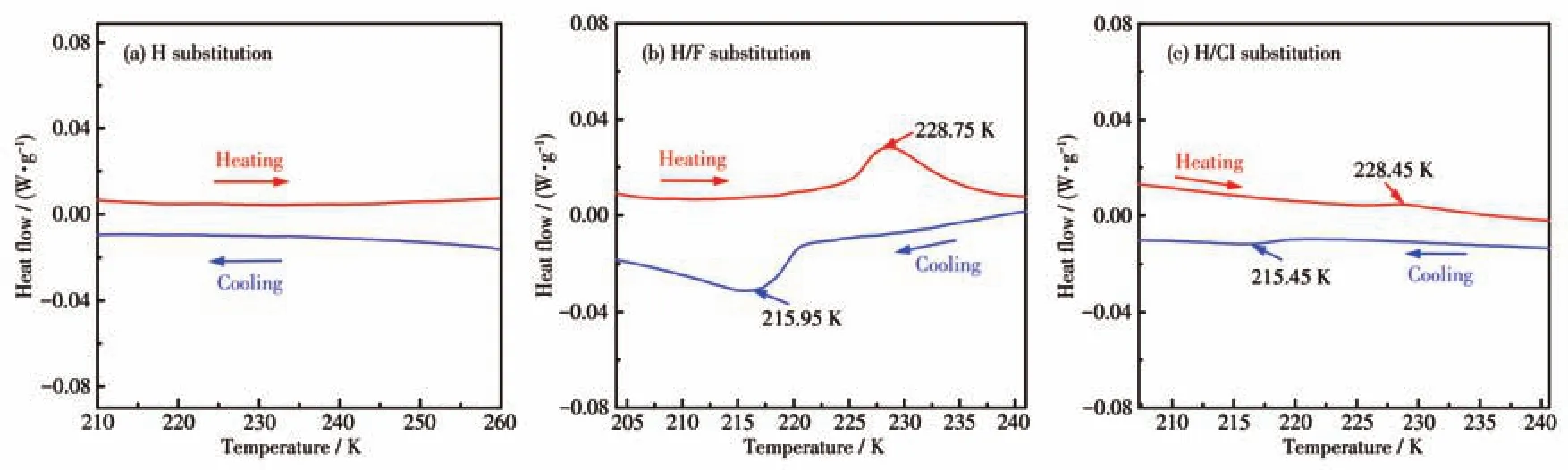
Fig.1 DSC curves of 1(a),2(b)and 3(c)obtained in a heating-cooling mode
2.2 Variable-temperature single crystal structure characterization
As for compound 1,the variable-temperature XRD was performed at 295 K.Compound 1 crystallizes in the monoclinicP21/n,witha=1.228 85(9)nm,b=0.928 18(6)nm,c=1.372 07(9)nm,β=110.457(8)°andV=1.466 28(19)nm3.The asymmetric unit of 1 is composed of one isolated[ZnBr4]2-tetrahedron and one(HEtHDabco)2+cation(Fig.2a).The pertinent crystallographic details of 1 are presented in Table S1.
To disclose the phase-transition mechanism,the single-crystal structures were obtained at 213 K and 293 K.Compounds 2 and 3 are isostructural,therefore,taking compound 2 as an example for showing structural details.At 213 K,it crystallizes in the orthorhombicPbca,witha=1.420 84(5)nm,b=1.426 50(9)nm,c=1.560 10(6)nm,andV=3.117 4(3)nm3.The structure is composed of an isolated[ZnBr4]2-tetrahedral and one(F-EtHDabco)2+cation(Fig.2c).The Zn atom coordinates with four terminal Br atoms,with Zn—Br bond distances varying from 0.243 68 to 0.238 9 nm and Br—Zn—Br bond angles ranging from 104.10(6)°to 113.04(7)°,which proves the distortion of the tetrahedral(Table S5).The(F-EtHDabco)2+cations,which reside in the cavities of the anionic framework,show a completely ordered state(Fig.3).Upon heating to 293 K,the cell parameters almost the same as that at RTP,namely,compound 2 undergoes an isostructural phase transition.The major change is that the(F-EtHDabco)2+achieves disordered configuration(Fig.2b),where C5,C6 and F1 atoms are split into two disordered sites as C5,C5′,C6,C6′and F1,F1′with occupancies of 0.5.As shown in Fig.3a and 3b,the packing diagrams of the two phases are similar,and the only difference is the disordered motion of cations.The results indicate that the order-disorder transformation of the(F-EtHDabco)2+cation is the major driving force for the phase transition.To explore the displacement of the inorganic anions in-depth,the schematic representations of[ZnBr4]2-are illustrated in Fig.3c and 3d.It is worth mentioning that the Zn…Zn distances change from 0.709 4~0.828 8 nm to 0.713 6~0.833 0 nm.Based on the above analyses,the order-disorder transformation of organic ammonium and the displacement of the inorganic framework together result in a dielectric response.
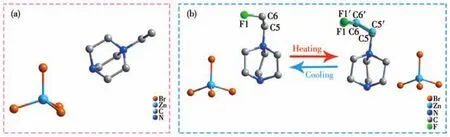
Fig.2 (a)Asymmetric unit of compound 1;(b)Asymmetric unit of compound 2 in LTP and RTP
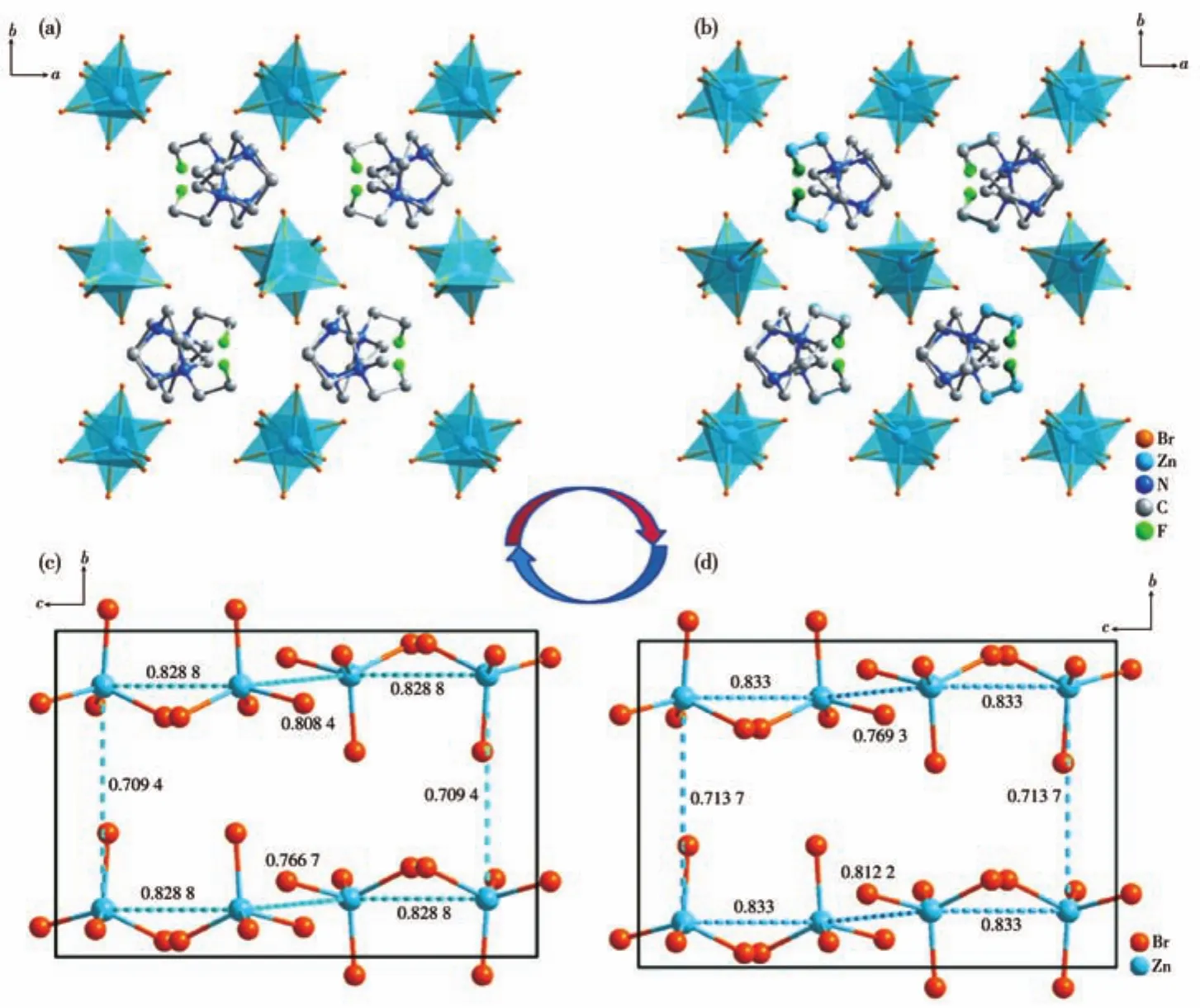
Fig.3 Projection of crystal structures of 2 in the LTP(a)and RTP(b)with the same orientations of the structures being taken for comparison;Distribution of[ZnBr4]2- in the LTP(c)and RTP(d)
To further analyze the differences in physical properties and structures of the three compounds,both Hirshfeld surfaces and two-dimensional(2D)fingerprint plots of the organic cations are discussed(Fig.4a~4c).By incorporating halogen atoms into(H-EtHDabco)2+,the H…Br interactions in compound 1 changed into X…Br(X=F,Cl)interactions.Obviously,the molecular interactions between(H-EtHDabco)2+and[ZnBr4]2-of 1 are stronger than that of 2 and 3.Therefore,there is no phase transition behavior of 1 in the studied temperature range.As shown in Fig.4b and 4c,the Br…F interaction in 2 was similar with the Br…Cl interaction in 3(red areas),indicating the analogous intermolecular interaction,which further proves the similar structure and properties of 2 and 3.
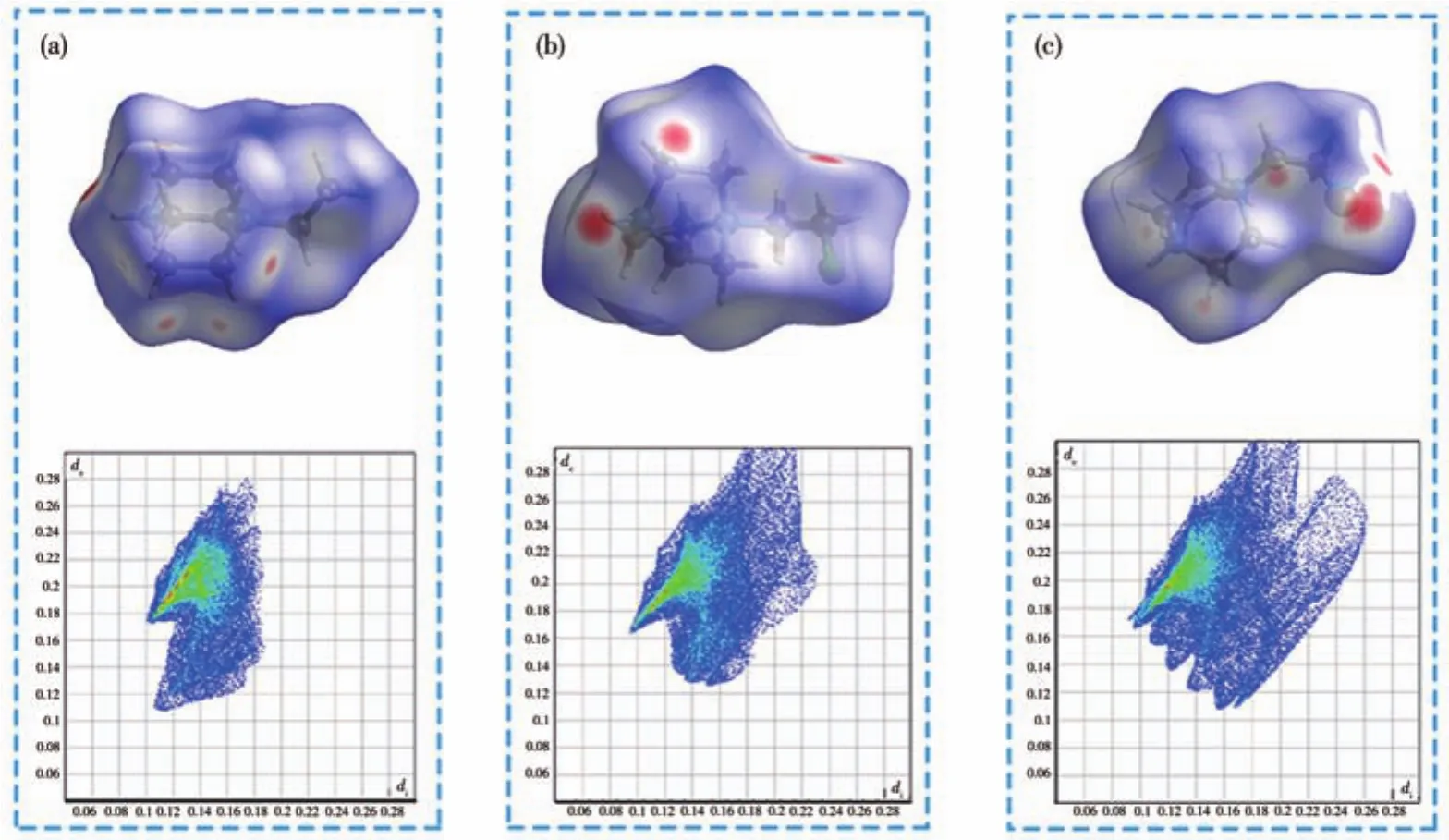
Fig.4 Hirshfeld dnorm surfaces and 2D fingerprint plots for 1(a),2(b)and 3(c)at 293 K
2.3 Dielectric properties
Generally,the dielectric response at variable temperature indicates thermally activated molecular rotations and structural changes.Specifically,the dielectric constantε(ε=ε′-iε″,whereε′is the real part andε″stands for the imaginary part)could act as a signal to judge the existence of structural phase transitions.As illustrated in Fig.5a,a pair of slight dielectric anomalies of 2 increased slowly from 4.40 to 4.67 in the vicinity ofTtr.For 3,the values ofε′varied from 6.57 and 6.85(Fig.5b).Furthermore,theε′-Tcurves at all measured frequencies showed that the positions ofTtrdid not move progressively towards higher temperatures with increasing frequency(Fig.5c,5d),that is to say,there was no frequency dependent for the dielectric response of 2 and 3.As shown in Fig.S4,the dielectric loss(ε″)of 2 and 3 displayed clear peak-like anomalies during the heating and cooling processes at 1 MHz,which further confirms the occurrence of the phase transition.
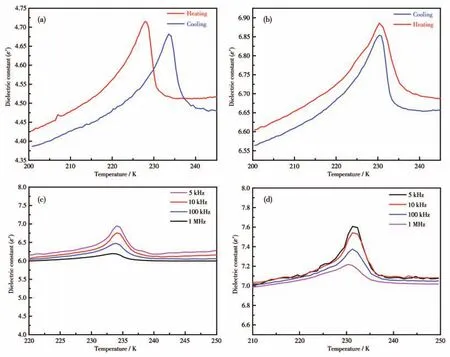
Fig.5 Temperature-dependent real part(ε′)of dielectric constant measured at 1 MHz for 2(a)and 3(b);Temperature-dependent dielectric constant(ε′)of frequencies from 5 kHz to 1 MHz upon cooling for 2(c)and 3(d)
3 Conclusions
In summary,a class of materials(H-EtHDabco)[ZnBr4](1),(F-EtHDabco)[ZnBr4](2)and(Cl-EtHDabco)[ZnBr4](3)were successfully synthesized and characterized.The results of DSC and dielectric measurements confirm the successful regulation to realize dielectric properties by replacing a hydrogen atom with a halogen atom(F or Cl).The order-disorder transformation of organic ammonium cations and displacement of[ZnBr4]2-anions both serve as the driving force for the reversible isostructural phase transition.This work provides an effective strategy for obtaining switchable material through precise chemical design.
Acknowledgments:This work was financial supported by the National Natural Science Foundation of China (Grant No.21991141).
Conflict of interest:The authors declare that they have no conflict of interest.
Supporting information is available at http://www.wjhxxb.cn
- 無機化學學報的其它文章
- Synthesis and Characterization of Palladium Nanoparticles with High Proportion of Exposed(111)Facet for Hydrogenation Performance
- Syntheses,Crystal Structures,Luminescence and Catalytic Activity of Manganese(Ⅱ)and Cadmium(Ⅱ)Coordination Polymers Based on 2,3-Dihydroxy-terephthalic Acid
- Scale-Up Strategy to Develop Highly-Effective Co-N-C@KB Composites as Sulfur Host for Lithium-Sulfur Battery
- Self-Assembled Zn2+,Co2+ and Ni2+ Complexes Based on Coumarin Schiff Base Ligands:Synthesis,Crystal Structure and Spectral Properties
- Synthesis,Characterization,and X-ray Crystal Structure Analysis of Cu(Ⅰ)/Cu(Ⅱ)Complexes of Phenanthridine and Triphenylphosphine
- 添加叔丁醇鉀對Mg(NH2)2-2LiH體系儲氫性能的影響

