A rational design of bimetallic PdAu nanoflowers as efficient catalysts for methanol oxidation reaction*
Jinyang Liu(劉錦陽), Min Wu(武敏), Xinyi Yang(楊新一),?,Juan Ding(丁娟), Weiwei Lei(類偉巍), and Yongming Sui(隋永明)
1State Key Laboratory of Superhard Materials,College of Physics,Jilin University,Changchun 130012,China
2Zhuhai College,Jilin University,Zhuhai 519041,China
3Institute for Frontier Materials,Deakin University,Geelong,Victoria,3216,Australia
Keywords: PdAu alloy,methanol oxidation reaction,catalyst
1. Introduction
Direct methanol fuel cell(DMFC),which is an emerging energy for portable and transport applications, has attracted much attention due to its high energy density and environmentally friendly.[1]Pt and Pt-based catalysts as the best catalysts have been widely applied in the methanol oxidation reaction(MOR) that is the anodic reaction of DMFC.[2]However, Pt and Pt-based catalysts are not only high cost but also easily poisoned by CO[3]which is generated in catalytic processes.Pd and Pd-based catalysts as a replacement attract more and more attention because of their low price.[4]And recent research,which could be a strong reason to explore Pd and Pdbased catalysts,shows that there is no evidence of CO formation for Pd-based catalysts in an alkaline environment.[5]
There are two ways to synthesize nanocatalysts with a high catalytic performance. One way is based on bifunctional reaction mechanism[6]and surface ligand effect.[7]In this way, one metal is doped with another metal like noble metal (Au,[8]Pt,[9]and Ru[10]) or transition metal (Ni,[11]Co,[12]and Cu[13]). Enhancement of the catalytic activity can attribute to the addition of different metal, which causes the shift of the d-band center of Pd with respect to the Fermi level in the alloys.[14]The other way is shape-controlled metal nanocrystals, such as wires,[8]icosahedrons,[15]cubes,[16]rob,[17]decahedrons,[18]and dendrites.[19]All of these show a high electrocatalytic activity because of high surface-tovolume ratio, high index facets, and lattice strain effects.[20]What is more, Han et al.[21]and Feng et al.[22]reported that they integrated the ways of the above mentioned to design and prepare three-dimensional multi-element alloy catalysts with superior catalytic activity for alcohol oxidation, which supplied a new way to design nanocatalysts with a high catalytic performance. Now, some strategies have been applied to design and optimize materials with enhancing catalytic performances, including seed mediated growth,[23]chemical vapor deposition,[24]and selective etching.[25]But these methods are often complicated and time consuming, which restrict their further applications.
In this work, we propose a simple way to synthesize flower-liked bimetallic PdAu nanocatalysts with controllable Pd/Au molar ratios. The as-prepared PdAu nanoflowers are self-supported porous structures with a high surface area. Especially, all of the PdAu nanoflowers catalysts show higher catalytic activities for MOR than commercial Pd/C catalyst in an alkaline environment due to their particular porous architectures and the shift of the d-band center of Pd.
2. Experimental details
Chemical reagent Palladium (II) sodium chloride(NaPdCl4),chloroauric acid(HAuCl4),and polyvinyl pyrrolidone(PVP,Mw≈40000)were purchased from Sigma-Aldrich.Citric acid, ethyl alcohol absolute, methanol (CH3OH),sodium hydroxide(NaOH),and sodium borohydride(NaBH4)were purchased from Sinopharm Chemical Reagent Company.Nafion 117 solution (5 wt%) and commercial Pd/C were ordered from Alfa Aesar. All the chemicals and materials were used as received.
Synthesis of PdAu nanoflowers catalysts To synthesize the PdAu nanoflowers catalysts,11 mg citric acid and 100 mg PVP were mixed with 30 mL deionized water(18.2 M?·cm),then it was injected with the mixture of 0.2 mM NaPdCl4(37.5μL)and 0.24 mM HAuCl4(31μL)under magnetic stirring.The 2.1 mg NaBH4was dissolved in 3 mL deionized water and subsequently added to the reaction mixture, followed by stirring for 30 min at room temperature. Finally, the suspensions were filtered and washed several times with deionized water and ethyl alcohol absolute to completely remove all excess reducing agent. Control experiments were performed by changing the molar ratio of NaPdCl4and HAuCl4, while the other experimental conditions were remained unchanged.
3. Characterization
Materials characterization Transmission electron microscopy (TEM), STEM-element mapping, and highresolution transmission electron microscopy (HRTEM) images of the simples were obtained on JEM2200FS with an accelerating voltage of 200 kV. X-ray diffraction was tested on Rigaku with a high-intensity microfocus rotating anode x-ray generator of MicroMax-007HF(λ =1.5418 ?A).
Electrochemical characterization All the electrochemical measurements were carried out on a three-electrode system on CHI-660E electrochemical workstation at room temperature. The sample and commercial Pd/C were applied to the surface of glassy carbon (GC) electrodes. Pt wire and saturated calomel electrode(SCE)served as the counter electrode and reference electrode,respectively. The electrochemical oxidation of methanol was performed in the mixture of 1.0 M NaOH containing 1.0 M CH3OH solution. The electrolyte solution was deoxygenated with N2for 15 min before measurement.
4. Results and discussion
4.1. Characterization of Pd1Au1 nanoflowers catalysts
The morphology and size of Pd1Au1nanoflowers catalysts were characterized by TEM.Figures 1(a)and 1(b)show the TEM images of Pd1Au1nanoflowers catalysts in different magnification,whose average diameter is ca.25 nm(insert in Fig. 1(a)). Besides, SAED pattern with obvious diffraction rings indicated its polycrystalline property. The surface structure of Pd1Au1nanoflowers catalysts was characterized by HRTEM in Fig.1(c). An individual Pd1Au1catalyst displays an obvious flower shape with porous architecture assembled by staggered nanocrystals as building blocks. Constructing open porous structure is of great importance to drastically suppress the activity loss derived from the agglomeration of active sites.[26,27]The lattice spacing distance of ca.0.227 nm can be observed clearly on the surface of Pd1Au1nanoflowers catalysts,which is between the interplanar spacing value 0.224 nm of Pd(111)plane and 0.235 nm of Au(111)plane.Figure 1(d)displays the elemental mapping of Pd,Au and their overlap for the Pd1Au1nanoflowers catalysts. Their overlap image can be seen clearly in elemental mapping patterns,which is a strong evidence for the generation of PdAu alloy (the signals of Pd in green and Au in yellow). And the chemical composition of Pd1Au1nanoflowers catalysts is characterized by EDS analysis to be 56:44 (Pd:Au), which is very close to the ratio of Pd:Au. Figures 2(a) and 2(b) show the TEM of PdAu2and PdAu3nanoflowers catalysts, respectively, indicating that the morphologies of PdAu nanoflowers catalysts are independent of the molar ratio of NaPdCl4and HAuCl4.
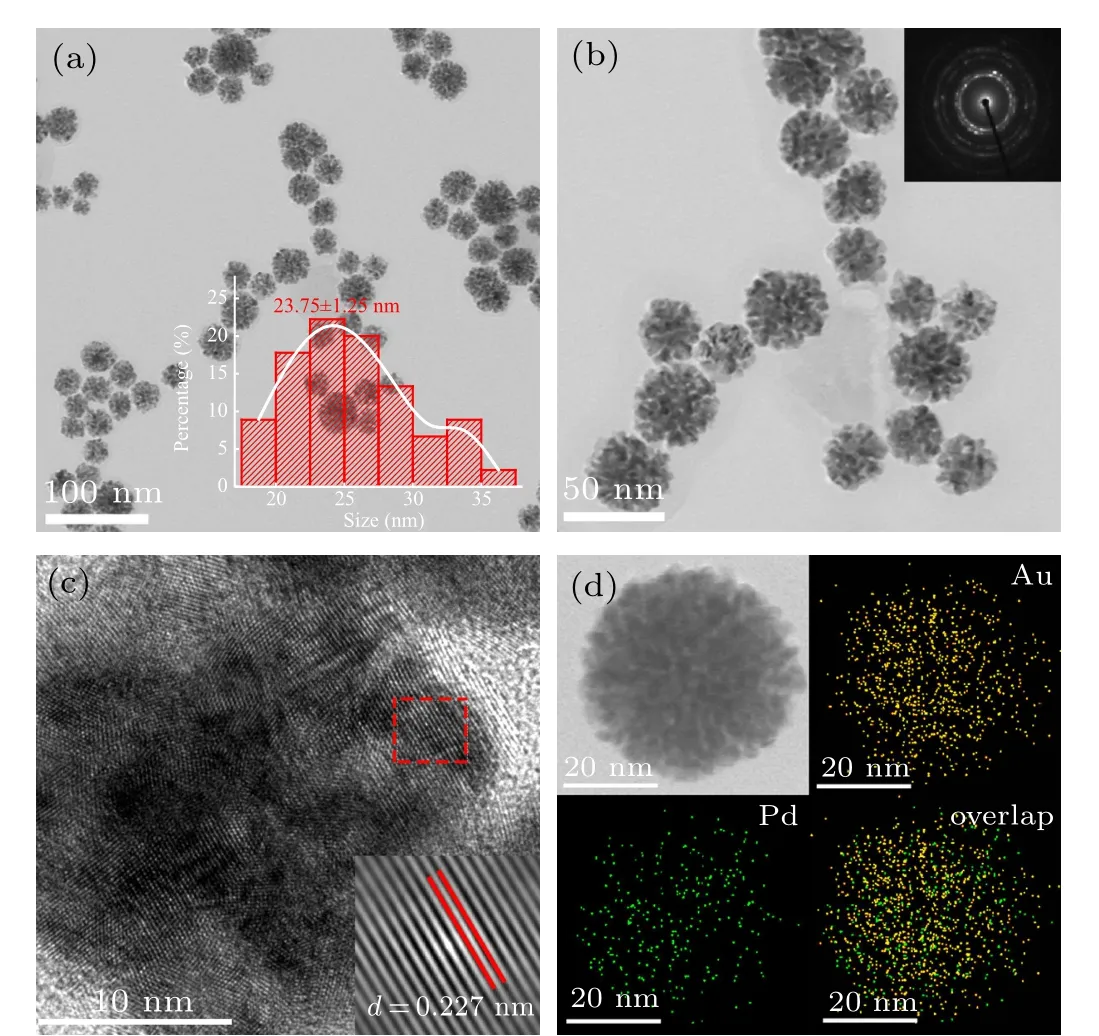
Fig. 1. (a) Low-magnification and (b) middle-magnification TEM images with SAED image of Pd1Au1 nanoflowers catalysts. (c) HRTEM image of Pd1Au1 nanoflowers catalysts. (d) HAADF-STEM-EDS mapping of Pd1Au1 nanoflowers catalysts.
To understand the impact of NaBH4as reductant on the growth of PdAu nanoflowers,we monitor the change of sample morphology by TEM.As shown in Fig.3(a),a large number of dispersed nanoparticles are produced. After injecting 40 μL NaBH4, both nanoparticles and nanodendrites can be simultaneously observed, indicating that branches grow from the nanoparticles (Fig. 3(b)). When injecting 100 μL NaBH4,the nanodendrites begin to clump together to generate nanoflowers(Fig.3(c)). With injecting NaBH4up to 200μL,the nanoparticles completely form nanoflowers (Fig. 3(d)).The morphology evolution with the addition of NaBH4gives an experimental evidence on the formation process of PdAu nanoflowers.
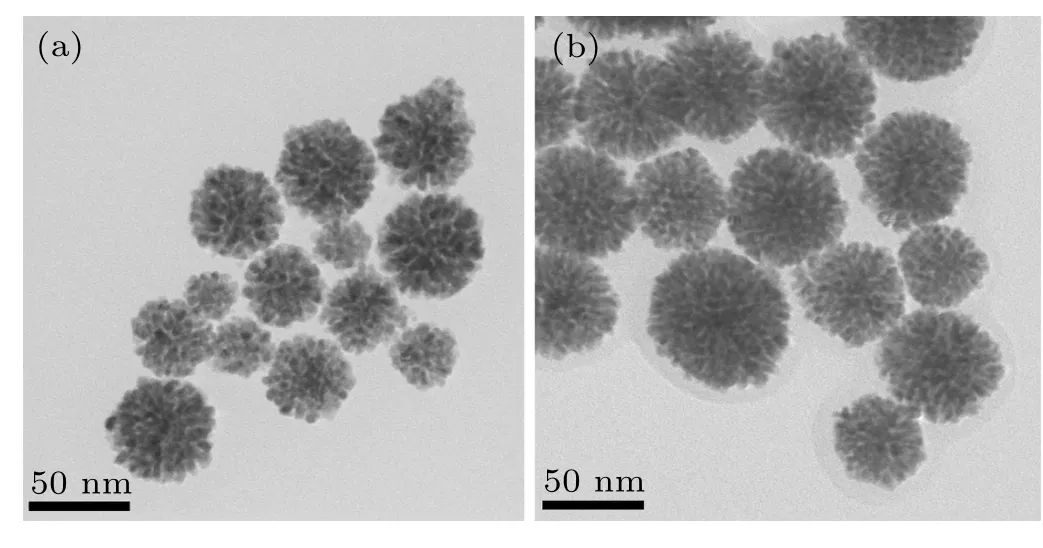
Fig.2. The TEM of morphologies of(a)PdAu2 and(b)PdAu3.
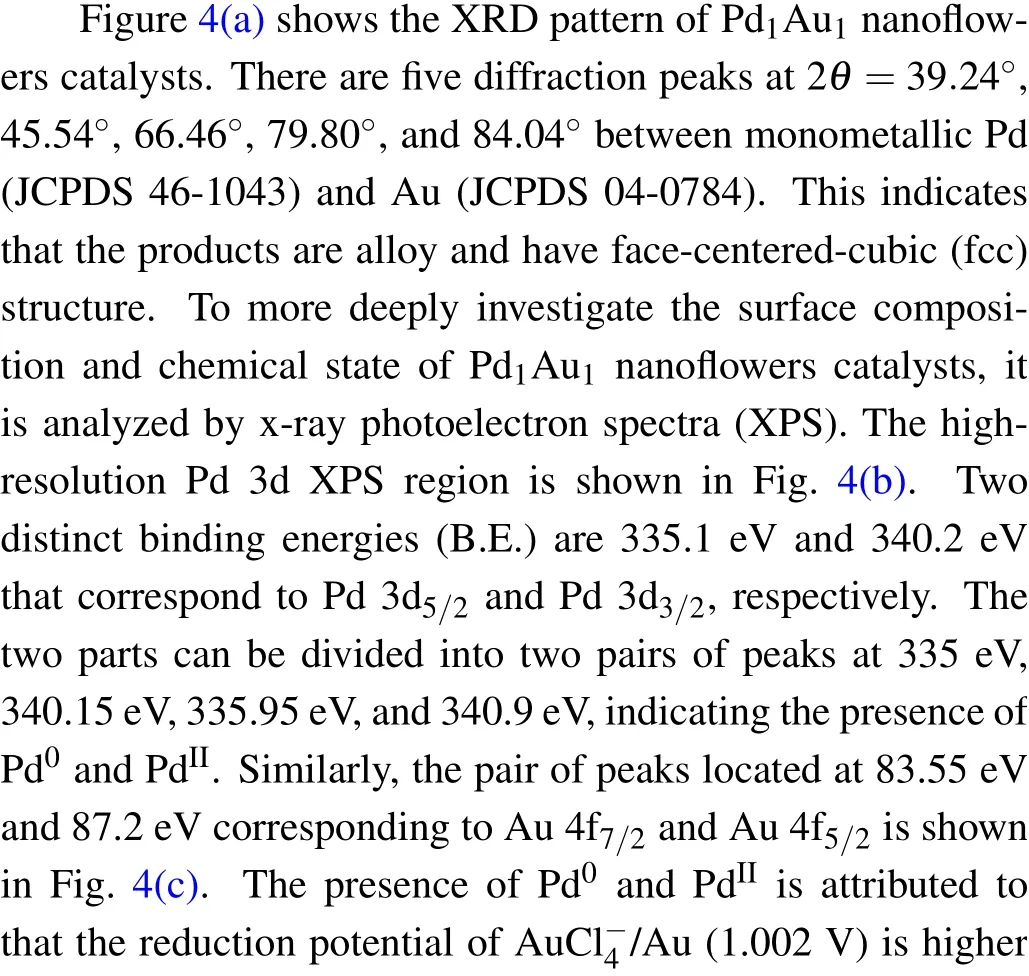
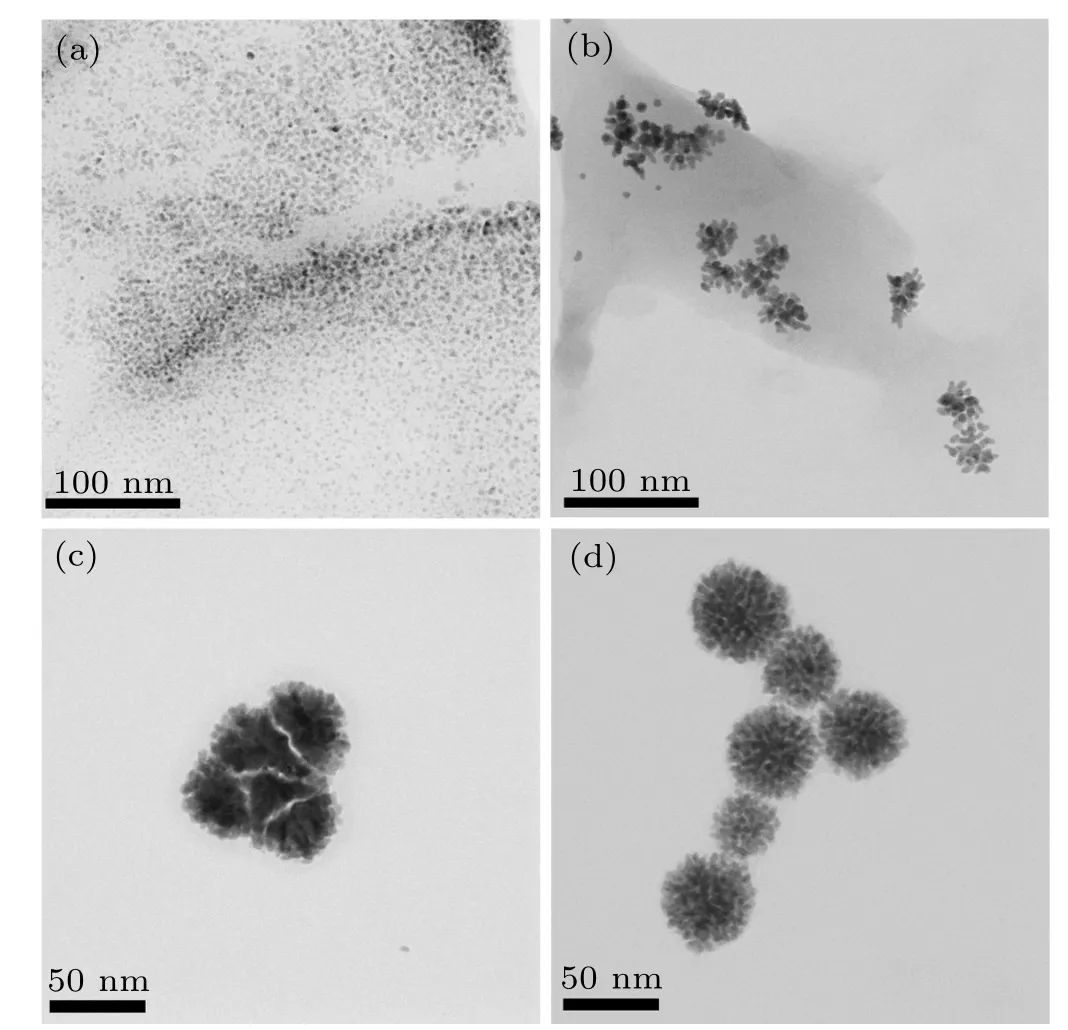
Fig.3. TEM of morphologies of PdAu after injecting(a)20μL,(b)40μL,(c)100μL,and(d)200μL NaBH4.

Fig.4. (a)XRD,(b)Pd 3d XPS,and(c)Au 4f XPS spectra of Pd1Au1 nanoflowers catalysts.
4.2. Electrochemical properties of PdAu nanoflowers catalysts
Figure 5(a) shows the cyclic voltammograms of Pd/C,Pd2Au, Pd1Au1, PdAu2, and PdAu3nanoflowers catalysts measured in 1.0 M KOH solution at a scan rate of 50 mV/s.During the negative scans, the peaks locate at ?0.1 V to?0.4 V can be observed for all catalysts,which are associated with the Pd oxide reduction. The peaks of Pd oxide reduction for Pd/C,Pd2Au,Pd1Au1,PdAu2,and PdAu3nanoflowers catalysts are located at around ?0.36 V,?0.28 V,?0.26 V,?0.2 V,and ?0.19 V,respectively. These peaks exhibit a shift from low potential to high potential,which is associated with decrease of Pd content in the PdAu alloy,proving that the catalytic activity of Pd is modified by Au. The electrochemical active surface area(ECSA)is calculated from the C–V curves according to the equation ECSA=Q/C. In this equation, Q is the charge from integrating the peaks of Pd oxide reduction and C is 4.2 C/m2, which is a constant charge required to reduce the monolayer of PdO adsorbed on the catalyst.
MOR activity of PdAu nanoflowers catalysts is tested in mixed solution of 1 M KOH and 1 M methanol at a scan rate of 50 mV/s. As shown in Fig.5(b),the current density is normalized by ECSA,we can see that the C–V curves of methanol oxidation contain forward scan and backward scan. One peak located in the forward scan is associated with methanol oxidation, the other is attributed to the removal of by-production such as formate produced in forward scan. In the forward scan, the onset potential (E0), peak potential (EP), and peak current density (jP) of catalysts are obtained from Fig. 5(b)and listed in Table 1. It indicates that alloying of Au with Pd can enhance the kinetics and improve the catalytic activity for methanol oxidation. The E0for PdAu nanoflowers catalysts is more negative than that of commercial Pd/C catalyst.With the decreasing Pd content, the EPshifts from negative potential to positive potential and jPfirst increases and then decreases. The EPand jPreach a maximum at PdAu (1:1).This phenomenon is due to that Au has insignificant electrochemical activity for methanol oxidation. Specific activity(SA) is shown in Fig. 5(c), which is calculated from the forward peak currents as normalized by ECSA. From Fig. 5(c),the Pd1Au1nanoflowers catalyst has the maximum SA,which is 0.72 mA/cm2,about 14 times that of commercial Pd/C catalyst. Others also show higher SA than commercial Pd/C catalyst.The excellent performance of PdAu nanoflowers catalysts could be attributed to the addition of Au,which makes the dband center of Pd down shift more obviously and accelerates the reaction of methanol oxidation.

Table 1. The E0,EP, jP,ECSA obtained from Fig.3.
Since the PdAu nanoflowers catalysts have similar characteristics with other Pd and Pd-based catalysts, we compare our catalysts with some commonly reported catalysts. For example, Luo et al.[28]provided SA of the Pd-black catalysts,which is 0.32 mA/cm2. Wang et al.[29]reported that the incorporation of Ag into Pd can enhance the MOR activity of Pd,which can be increased to 0.68 mA/cm2(Pd:Ag=1:1)and 0.71 mA/cm2(Pd:Ag=1:1.5).Liu et al.[30]showed that the porous morphology can improve the MOR specific activity of PdAu catalysts,whose best SA is 0.27 mA/cm2. It can be seen that the Pd1Au1nanoflowers catalysts are competitive and outstanding for methanol oxidation reaction compared with other catalysts.
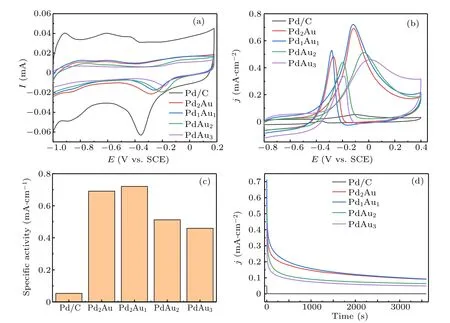
Fig.5. Cyclic voltammograms of Pd/C,Pd2Au,Pd1Au1,PdAu2,and PdAu3 catalysts in(a)1 M KOH and(b)1 M KOH+1 M CH3OH.(c)Specific activities. (d)Chronoamperograms of Pd/C,Pd2Au,Pd1Au1,PdAu2,and PdAu3 nanoflowers catalysts.
To evaluate the electrocatalytic endurance of PdAu nanoflowers catalysts,chronoamperometric experiment has be performed at ?0.12 V and the results are shown in Fig.5(d).All of the catalysts show a significant current decrease at first and then gradually slow down and level off. During 1 h test,all of the PdAu nanoflowers catalysts have higher current density than commercial Pd/C catalyst. And Pd1Au1nanoflowers catalyst shows the highest current density at 3600 s. From Fig. 5(d), we can infer that doping Au into Pd can effectively improve the catalyst stability toward methanol oxidation. What is more, the Pd1Au1nanoflowers catalyst is the most superior catalyst among the catalysts investigated,which can illustrate that the appropriate incorporation of Au into Pd can effectively promote the reduction of PdO back to Pd to improve the stability of catalyst.
5. Conclusion
In summary, we develop a simple aqueous synthetic method to prepare bimetallic PdAu alloy nanoflowers. The prepared Pd1Au1nanoflowers show the best specific activity and stability toward methanol oxidation reaction, compared with other PdAu nanoflowers catalysts and commercial Pd/C catalyst. The main reason of the enhanced electrocatalytic performance is that Au could modify the d-band center of Pd. We hope that this work could be used in designing multi-component and unprecedented electrocatalysts for direct methanol fuel cells.
- Chinese Physics B的其它文章
- Process modeling gas atomization of close-coupled ring-hole nozzle for 316L stainless steel powder production*
- A 532 nm molecular iodine optical frequency standard based on modulation transfer spectroscopy*
- High-throughput identification of one-dimensional atomic wires and first principles calculations of their electronic states*
- Effect of tellurium(Te4+)irradiation on microstructure and associated irradiation-induced hardening*
- Effect of helium concentration on irradiation damage of Fe-ion irradiated SIMP steel at 300 °C and 450 °C*
- Optical spectroscopy study of damage evolution in 6H-SiC by H+2 implantation*

