組蛋白乙酰化修飾調(diào)控NF-κB驅(qū)動的PNPLA3基因表達的機制初探
許曉?陳蕓芝?徐芬?梁華
【摘要】目的 探討禁食/再喂食后小鼠肝臟Patatin樣磷脂酶域蛋白3(PNPLA3)基因啟動子核因子-κB(NF-κB)結(jié)合區(qū)域組蛋白H3K9乙酰化(H3K9ac)水平以及相關去乙酰化酶(HDAC)和乙酰化轉(zhuǎn)移酶(HAT)的變化特點。方法 根據(jù)體質(zhì)量將18只C57BL/6小鼠隨機分為3組各6只,分別為自由飲食組、禁食組(夜間饑餓24 h)和再喂食組(饑餓24 h后再自由攝食高蔗糖含量飼料12 h),分別予自由飲食、禁食和禁食后再喂食干預,檢測并比較各組肝臟PNPLA3、NF-κB、HDAC(SIRT1、SIRT6)和HAT(GCN5、Elp3)基因表達情況,用染色質(zhì)免疫共沉淀-定量PCR檢測H3K9ac富集水平,并分析H3K9ac與PNPLA3表達的相關性。結(jié)果 3組比較,C57BL/6小鼠肝臟PNPLA3、NF-κB基因表達在禁食時下調(diào),再喂食后又上調(diào)(P均< 0.001)。H3K9ac水平變化與PNPLA3變化趨勢一致(P均< 0.05),相關分析提示兩者具有相關性(rs = 0.958, P < 0.001);禁食組SIRT1和SIRT6表達水平較自由飲食組高,再喂食組兩者水平均下降(P均< 0.05),與H3K9ac變化趨勢相反;GCN5、Elp3表達變化與SIRT1及SIRT6類似(P均< 0.05)。結(jié)論 SIRT1和SIRT6相關H3K9去乙酰化涉及飲食調(diào)節(jié)NF-κB驅(qū)動的PNPLA3的表達。
【關鍵詞】Patatin樣磷脂酶域蛋白3;組蛋白 H3K9 乙酰化;禁食/再喂食;核因子-κB
Preliminary study of the mechanism of histone acetylation modification in regulating NF-κB-driven PNPLA3 gene expression in liver Xu Xiao, Chen Yunzhi, Xu Fen, Liang Hua. Department of Endocrinology and Metabolism, the Third Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Diabetology, Guangzhou? 510630, China
Corresponding author, Liang Hua, E-mail: lianghua@ mail. sysu. edu. cn
【Abstract】Objective To characterize the changes in the levels of H3K9 acetylation (H3K9ac) around the Nuclear factor-κB (NF-κB) binding site of PNPLA3 gene promoter and the expression levels of related deacetylase (HDAC) and acetyltransferase (HAT) enzymes in the liver of C57BL/6 mice during the fasting/re-feeding transition. Methods According to the weight, 18 C57BL/6 mice were randomly assigned into the ad libitum feeding group (n = 6), fasting group (n = 6, fasting for 24 h during night) and re-feeding group (n = 6, 24-h fasting followed by ad libitum re-feeding with high sucrose diet for 12 h). Mice were subjected to fasting/re-feeding interventions. The expression levels of PNPLA3, NF-κB, HDAC (SIRT1, SIRT6) and HAT (GCN5, Elp3) genes were detected and compared among three groups. The H3K9ac enrichment level was assayed by using ChIP-qPCR. Correlation analysis was performed to analyze the relationship between H3K9ac and PNPLA3 expression levels. Results The expression levels of PNPLA3 and NF-κB genes in C57BL/6 mouse liver were significantly down-regulated during fasting and then significantly up-regulated after re-feeding (all P < 0.001). The changes in the H3K9ac expression level were consistent with those in PNPLA3 (all P < 0.05). Correlation analysis suggested a significant association between H3K9ac and PNPLA3 expression levels (rs = 0.958, P < 0.001). The expression levels of SIRT1 and SIRT6 in the fasting group were significantly higher compared with those in the ad libitum feeding group, which were significantly down-regulated in re-feeding groups (all P < 0.05), contrary to the changing trend of H3K9ac. The changes in the expression levels of GCN5 and Elp3 were similar with those of SIRT1 and SIRT6 (all P < 0.05). Conclusions SIRT1 and SIRT6-associated H3K9 deacetylation may be involved in the dietary regulation of NF-κB-driven PNPLA3 expression.
【Key words】Patatin-like phospholipase-domain-containing 3;H3K9 acetylation;
Fasting/re-feeding;Nuclear factor-κB
非酒精性脂肪肝(NAFLD)與飲食關系密切。近年來有研究顯示,頻繁重復的長時間禁食(間歇禁食)具有改善NAFLD的作用,但具體分子機制尚未明確[1-2]。NAFLD是復雜性狀疾病,其發(fā)病是遺傳與環(huán)境因素共同作用的結(jié)果,表觀遺傳學修飾是在不改變DNA序列的情況下環(huán)境調(diào)控基因表達的關鍵環(huán)節(jié)[3]。既往研究表明,飲食和營養(yǎng)可通過表觀遺傳改變?nèi)旧w結(jié)構和功能從而改變基因表達,形成多樣性生物表型[4]。因此我們推測,表觀遺傳學修飾是間歇性禁食啟動肝臟基因表達調(diào)控的關鍵。Patatin樣磷脂酶域3 (PNPLA3)主要表達于肝臟組織,是NAFLD的主要易感基因[5]。我們的前期研究顯示核因子-κB(NF-κB)調(diào)控PNPLA3表達參與PNPLA3基因 I148M多態(tài)性相關NAFLD炎癥進展[6]。在人類和動物實驗中,禁食可改善NAFLD炎癥狀態(tài),其機制是否涉及表觀遺傳學修飾調(diào)控NF-κB驅(qū)動的PNPLA3表達尚不清楚[7-8]。因此本課題組擬在禁食/再喂食干預小鼠中初步探討肝臟PNPLA3、NF-κB基因表達,PNPLA3基因啟動子NF-kB結(jié)合區(qū)域組蛋白H3賴氨酸9乙酰化(H3K9ac)水平以及H3K9相關去乙酰化酶和乙酰化轉(zhuǎn)移酶的變化特點, 為揭示間歇性禁食改善NAFLD的機制奠定基礎。
材料與方法
一、動 物
7 ~ 8周齡雄性C57BL/6鼠18只,體質(zhì)量20 ~ 25 g,購自南京大學南京生物醫(yī)藥研究院。
二、試 劑
對照飼料(AIN93純化型標準飼料)以及高蔗糖飼料(65%蔗糖含量)購自南通特洛菲飼料科技有限公司(中國);染色質(zhì)免疫共沉淀(ChIP)試劑盒(貨號:17-10086) 購自Millipore公司(美國);動物組織RNA提取試劑盒(貨號:74134)購自QIAGEN(德國),定量PCR (qPCR) 試劑盒(貨號:04707516001)購自Roche公司(美國);逆轉(zhuǎn)錄PCR(RT-PCR)試劑盒5x All-In-One RT Master Mix (貨號:G490) 購自Abm公司(加拿大)。免疫共沉淀使用CST公司(美國)的anti-H3k9Ac抗體(9649s),陰性對照組rabbit lgG抗體(B900610)購自Proteintech(中國)。
三、方 法
1.小鼠自由飲食、禁食和再喂食干預
18只C57BL/6J小鼠分為3只/籠,共6籠,自由取水和飲食,環(huán)境溫度為22℃,濕度為60%。適應性喂養(yǎng)1周后,根據(jù)體質(zhì)量將其隨機分為3組各6只,分別為自由飲食組、禁食組(夜間饑餓24 h)和再喂食組(饑餓24 h后再自由攝食高蔗糖含量飼料12 h)。干預結(jié)束后留取小鼠肝臟組織進行后續(xù)檢測。本動物實驗倫理經(jīng)中山大學實驗動物管理與使用委員會審批通過(SYSU-IACUC-2020-000175)。
2. 染色質(zhì)免疫共沉淀-定量PCR(ChIP-qPCR)
從每組中取4只小鼠進行ChIP-qPCR,將
20 mg小鼠肝臟組織(大約含5×106個肝臟細胞)剪碎成1 mm3組織小塊后用1%甲醛交聯(lián),再使用勻漿儀將其研磨至單細胞狀態(tài),依次加入ChIP試劑盒提供的細胞裂解液及細胞核裂解液,解析出染色質(zhì),隨后使用Diagenode公司(比利時)的Bioruptor UCD-200非接觸式全自動超聲波破碎儀剪切染色質(zhì)至200 ~ 1000 bp,后續(xù)步驟嚴格按照ChIP試劑盒說明進行。免疫共沉淀用anti-H3k9Ac抗體,使用兔lgG抗體作為陰性對照。提純的DNA使用qPCR試劑盒進行擴增定量,所用正向引物:5-GGAAAGACAGCATGTGGATGGT-3,反向引物:5-GATGCTTGCTGGGCAGAATG-3,產(chǎn)物覆蓋PNPLA3啟動子的NF-κB結(jié)合區(qū)域。用占input的百分比(% input)來表示免疫沉淀樣品中H3K9ac在PNPLA3啟動子NF-κB結(jié)合區(qū)域的富集度。
3. RT-PCR和qPCR
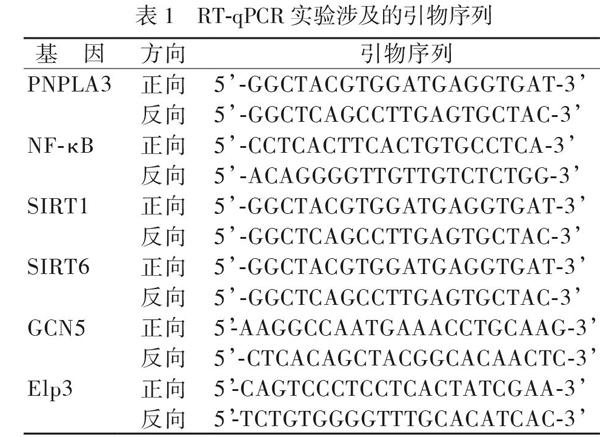
為檢測肝臟PNPLA3、NF-κB、H3K9相關去乙酰化酶(SIRT1、SIRT6)、乙酰化酶(GCN5、Elp3)mRNA水平,使用組織RNA提取試劑盒,提取每組所有小鼠肝臟RNA,使用Nanadrop2000分光光度計測定總RNA濃度和純度,OD260/280、OD260/230在1.9 ~ 2.1提示純度較高。使用ABM公司試劑盒進行RT-PCR,反應條件為:25℃ 10 min,42℃ 15 min,85℃ 5 min,反應產(chǎn)物為互補DNA(cDNA)。以cDNA為模板,使用試劑盒進行qPCR實驗, 反應條件:90℃ 30 s,95℃ 5 s,60℃ 20 s,40個循環(huán); 95℃ 5 s,65℃ 15 s,95℃ continuous;40℃ 30 s。本研究所用qPCR引物序列如表1。
四、統(tǒng)計學處理
采用SPSS 20.0分析數(shù)據(jù),用GraphPad Prism 8.0作圖,各組數(shù)據(jù)均符合正態(tài)分布,計量資料用表示,多組定量資料比較用方差分析,兩兩比較用Dunnet-t法(比較自由飲食組和禁食組、禁食組和再喂食組)。相關分析中變量不符合正態(tài)分布,使用Spearman秩相關分析。P < 0.05 (雙側(cè))為差異有統(tǒng)計學意義。
結(jié)果
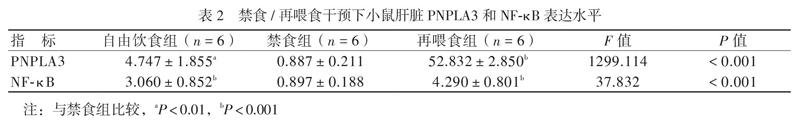
一、禁食/再喂食對小鼠肝臟PNPLA3和NF-κB表達的影響
禁食組小鼠肝臟PNPLA3表達水平較自由飲食組低,再喂食組PNPLA3表達水平最高。NF-κB在各組的表達趨勢與PNPLA3一致,即在禁食時表達下調(diào),禁食后再喂食表達上調(diào),見表2。
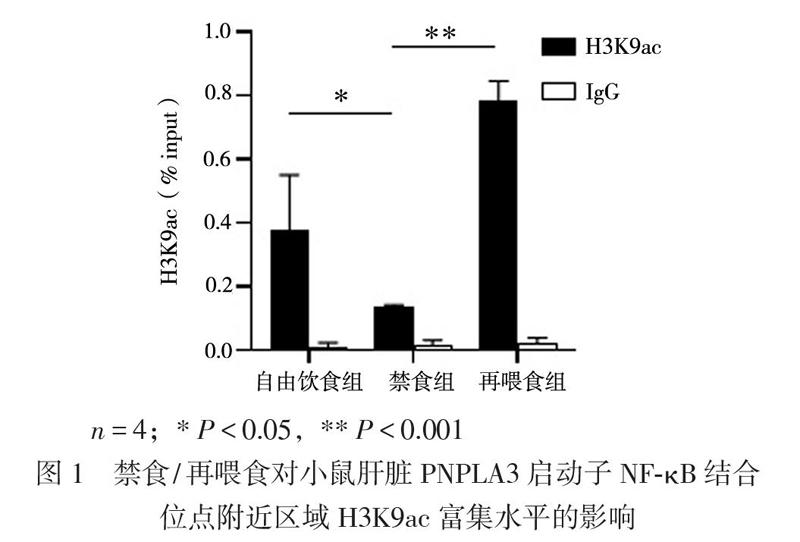
二、禁食/再喂食對小鼠肝臟PNPLA3啟動子NF-kB結(jié)合位點附近區(qū)域H3K9ac富集水平的影響
小鼠肝臟PNPLA3轉(zhuǎn)錄調(diào)控區(qū)域H3K9ac的水平在禁食/再喂食轉(zhuǎn)換過程中的變化與PNPLA3的表達趨勢一致,表現(xiàn)為禁食組H3K9ac水平較自由飲食組低(0.135±0.007 vs. 0.379±0.171,P < 0.05),再喂食組較禁食組高(0.780±0.064 vs. 0.135±0.007,P < 0.001),見圖1。相關分析顯示PNPLA3的基因表達和H3K9ac水平呈正相關
(rs = 0.958, P < 0.001)。
三、禁食/再喂食對小鼠肝臟H3K9ac 相關修飾酶表達的影響
禁食/再喂食時去乙酰化酶SIRT1以及SIRT6變化和H3K9ac的變化模式相反,均表現(xiàn)為禁食組表達水平較自由飲食組高(P < 0.05),再喂食組較禁食組低(P < 0.05),見圖2A、B。GCN5和Elp3是增加組蛋白乙酰化水平的乙酰化酶,在禁食/再喂食中GCN5和Elp3的表達特征與SIRT1和SIRT6類似,在禁食組中表達水平高(P < 0.05),在再喂食組中表達水平低(P < 0.05),與 PNPLA3啟動子H3K9ac水平的變化不相符,見圖2C、D。
討論
NAFLD與2型糖尿病、心血管疾病關系密切,是全球重要的公共健康問題之一,其治療方法主要是生活方式干預[9-10]。不斷增加的研究證據(jù)顯示,間歇禁食具有改善NAFLD的作用,但其作用機制尚未明確[1-2]。PNPLA3基因是NAFLD主要易感基因,其表達受禁食再喂食狀態(tài)調(diào)節(jié),提示PNPLA3可能參與間歇禁食改善NAFLD的機制[5, 11-12]。PNPLA3基因表達調(diào)控機制尚未被完全闡明。NF-κB等對PNPLA3表達的調(diào)控已被本課題組及其他研究者證實[6, 12]。環(huán)境因素對基因表達的調(diào)控是通過誘導基因表觀遺傳學修飾實現(xiàn)的。UCSC數(shù)據(jù)庫顯示人或小鼠肝臟和肝細胞PNPLA3基因啟動子存在超染色質(zhì)活性區(qū)域和H3K9ac等豐富的組蛋白乙酰化修飾。但組蛋白乙酰化修飾調(diào)控PNPLA3基因表達的機制尚未見報道。在本研究中,我們初步探討了禁食/再喂食時小鼠PNPLA3基因啟動子NF-κB結(jié)合區(qū)域附近H3K9ac水平隨能量狀態(tài)變化的特點,以及可能調(diào)控H3K9乙酰化和去乙酰化酶表達水平的變化,為了解環(huán)境與基因相互作用促進NAFLD發(fā)生發(fā)展提供實驗依據(jù),也為揭示間歇禁食改善NAFLD的機制提供了新思路。
我們的前期研究證實NF-κB是PNPLA3的直接轉(zhuǎn)錄因子[6]。在本研究中,NF-κB表達受營養(yǎng)狀態(tài)影響,禁食時表達降低,再喂食后表達增加,該結(jié)果與既往研究報道一致[8]。組蛋白乙酰化/去乙酰化可通過改變?nèi)旧|(zhì)結(jié)構影響轉(zhuǎn)錄因子和基因啟動子結(jié)合而調(diào)控基因轉(zhuǎn)錄。因此,我們檢測到的PNPLA3啟動子NF-κB結(jié)合區(qū)域H3K9ac的富集水平在禁食時下降可能導致染色質(zhì)結(jié)構致密而減弱NF-κB與PNPLA3啟動子結(jié)合,降低PNPLA3表達,提示了低H3K9乙酰化抑制NF-κB驅(qū)動的PNPLA3表達可能涉及間歇禁食改善NAFLD的機制。
組蛋白乙酰化修飾過程處于動態(tài)平衡,并由組蛋白乙酰化轉(zhuǎn)移酶和組蛋白去乙酰化酶共同調(diào)控。表觀遺傳學數(shù)據(jù)庫Histome數(shù)據(jù)庫(www.iiserpune.ac.in)及WERAM數(shù)據(jù)庫(http://weram.biocuckoo.org/)顯示,調(diào)控H3K9ac的去乙酰化酶為SIRT1、SIRT6,乙酰化酶為GCN5及Elp3。SIRT1和SIRT6表達下調(diào)是NAFLD發(fā)生發(fā)展的重要機制之一[13-15]。肝臟SIRT1和SIRT6表達也受營養(yǎng)狀態(tài)調(diào)控,我們發(fā)現(xiàn)SIRT1和SIRT6表達水平在禁食時升高,再喂食后表達水平降低,這與既往研究結(jié)果一致[15-16]。此外,禁食/再喂食中SIRT1和SIRT6表達變化趨勢與PNPLA3基因表達和PNPLA3啟動子 H3K9ac水平相反,提示SIRT1和SIRT6可能涉及PNPLA3啟動子H3K9ac水平調(diào)控。
GCN5和Elp3是H3K9ac相關乙酰化酶,涉及基因表達的增加。但在我們的研究中,禁食/再喂食時GCN5和Elp3表達表現(xiàn)出與預期(即禁食時降低再喂食后增加)相反的趨勢,提示GCN5和Elp3可能不是調(diào)控PNPLA3基因H3K9ac水平的乙酰化酶。既往報道GCN5主要參與糖異生基因表達的轉(zhuǎn)錄抑制,由于PNPLA3基因功能不涉及糖代謝,GCN5可能不涉及PNPLA3基因表達的表觀遺傳學調(diào)控機制[17]。Elp3的相關研究主要集中在腫瘤研究,在代謝領域較少,其在禁食/再喂食中表達的變化效應和機制尚需要更進一步的實驗來揭示。
綜上所述,禁食/再喂食可能影響H3K9ac水平,調(diào)控NF-κB驅(qū)動的PNPLA3表達,SIRT1和SIRT6可能是負責此調(diào)控作用的去乙酰化酶。在本研究中我們對PNPLA3基因表觀調(diào)控機制的探討尚淺,需進一步行體內(nèi)外研究,更深入探討SIRT1、SIRT6與H3K9ac之間的關系以及SIRT1和SIRT6對NF-κB與PNPLA3啟動子結(jié)合力及轉(zhuǎn)錄活性的影響。
參 考 文 獻
[1] Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, Chen RQ, Chen ZY. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol,2019,19(1):219.
[2] Aliasghari F, Izadi A, Gargari BP, Ebrahimi S. The effects of ramadan fasting on body composition, blood pressure, glucose metabolism, and markers of inflammation in NAFLD patients: an observational trial. J Am Coll Nutr,2017,36(8):640-645.
[3] Gallego-Durán R, Romero-Gómez M. Epigenetic mechanisms in non-alcoholic fatty liver disease: an emerging field. World J Hepatol,2015,7(24):2497-2502.
[4] Tiffon C. The impact of nutrition and environmental epigenetics on human health and disease. Int J Mol Sci, 2018,19(11):3425.
[5] Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet,2008,40(12):1461-1465.
[6] Yuan S, Liu H, Yuan D, Xu J, Chen Y, Xu X, Xu F, Liang H. PNPLA3 I148M mediates the regulatory effect of NF-κB on inflammation in PA-treated HepG2 cells. J Cell Mol Med,2020,24(2):1541-1552.
[7] Aliasghari F, Izadi A, Gargari BP, Ebrahimi S. The effects of ramadan fasting on body composition, blood pressure, glucose metabolism, and markers of inflammation in NAFLD patients: an observational trial. J Am Coll Nutr, 2017, 36(8):640-645.
[8] Sokolovi? A, van Roomen CP, Ottenhoff R, Scheij S, Hiralall JK, Claessen N, Aten J, Oude Elferink RP, Groen AK, Sokolovi? M. Fasting reduces liver fibrosis in a mouse model for chronic cholangiopathies. Biochim Biophys Acta,2013,1832(10):1482-1491.
[9] Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol,2011,9(6):524-530.
[10] Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care,2007,30(5):1212-1218.
[11] Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology,2011,53(6):1883-1894.
[12] Liang H, Xu J, Xu F, Liu H, Yuan D, Yuan S, Cai M, Yan J, Weng J. The SRE motif in the human PNPLA3 promoter (-97 to -88 bp) mediates transactivational effects of SREBP-1c. J Cell Physiol,2015,230(9):2224-2232.
[13] Xu F, Li Z, Zheng X, Liu H, Liang H, Xu H, Chen Z, Zeng K, Weng J. SIRT1 mediates the effect of GLP-1 receptor agonist exenatide on ameliorating hepatic steatosis. Diabetes,2014,63(11):3637-3646.
[14] Zheng X, Xu F, Liang H, Cao H, Cai M, Xu W, Weng J. SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum stress. Hepatology,2017,66(3):809-824.
[15] Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab,2010,12(3):224-236.
[16] Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L, Zang M. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology, 2014,146(2):539-549.
[17] Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab,2006,3(6):429-438.
(收稿日期:2020-11-25)
(本文編輯:洪悅民)

